Ecto-CD38-NADase inhibition modulates cardiac metabolism and protects mice against doxorubicin-induced cardiotoxicity
- PMID: 38271281
- PMCID: PMC10953800
- DOI: 10.1093/cvr/cvae025
Ecto-CD38-NADase inhibition modulates cardiac metabolism and protects mice against doxorubicin-induced cardiotoxicity
Abstract
Aims: Doxorubicin (DXR) is a chemotherapeutic agent that causes dose-dependent cardiotoxicity. Recently, it has been proposed that the NADase CD38 may play a role in doxorubicin-induced cardiotoxicity (DIC). CD38 is the main NAD+-catabolizing enzyme in mammalian tissues. Interestingly, in the heart, CD38 is mostly expressed as an ecto-enzyme that can be targeted by specific inhibitory antibodies. The goal of the present study is to characterize the role of CD38 ecto-enzymatic activity in cardiac metabolism and the development of DIC.
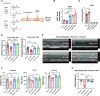
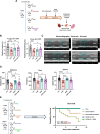
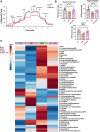
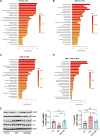
Methods and results: Using both a transgenic animal model and a non-cytotoxic enzymatic anti-CD38 antibody, we investigated the role of CD38 and its ecto-NADase activity in DIC in pre-clinical models. First, we observed that DIC was prevented in the CD38 catalytically inactive (CD38-CI) transgenic mice. Both left ventricular systolic function and exercise capacity were decreased in wild-type but not in CD38-CI mice treated with DXR. Second, blocking CD38-NADase activity with the specific antibody 68 (Ab68) likewise protected mice against DIC and decreased DXR-related mortality by 50%. A reduction of DXR-induced mitochondrial dysfunction, energy deficiency, and inflammation gene expression were identified as the main mechanisms mediating the protective effects.
Conclusion: NAD+-preserving strategies by inactivation of CD38 via a genetic or a pharmacological-based approach improve cardiac energetics and reduce cardiac inflammation and dysfunction otherwise seen in an acute DXR cardiotoxicity model.
Keywords: CD38; Cardiotoxicity; DIC; Doxorubicin; Ecto-NADase; Heart failure; NAD+.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: E.N.C. holds a patent on the use of CD38 inhibitors for metabolic diseases licensed by Elysium Health. E.N.C. is a consultant for TeneoBio, Calico, Mitobridge, and Cytokinetics. E.N.C. is on the advisory board of Eolo Pharma. E.N.C. and WVS own stocks in TeneoBio. Research in the E.N.C. laboratory has been conducted in compliance with Mayo Clinic’s conflict of interest policies.
Figures



References
-
- Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639–1642. - PubMed
-
- Wan Y, He B, Zhu D, Wang L, Huang R, Zhu J, Wang C, Gao F. Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Arch Biochem Biophys 2021;712:109050. - PubMed
-
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun 2006;349:353–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

