Use of isotretinoin among girls and women of childbearing age and occurrence of isotretinoin-exposed pregnancies in Germany: A population-based study
- PMID: 38271295
- PMCID: PMC10810459
- DOI: 10.1371/journal.pmed.1004339
Use of isotretinoin among girls and women of childbearing age and occurrence of isotretinoin-exposed pregnancies in Germany: A population-based study
Abstract
Background: Exposure to isotretinoin during pregnancy must be avoided due to its teratogenicity, but real-world data on its use are scarce. We aimed to describe (i) isotretinoin use in women of childbearing age in Germany; (ii) the occurrence of isotretinoin-exposed pregnancies; and (iii) malformations among children exposed in utero.
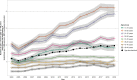
Methods and findings: Using observational data from the German Pharmacoepidemiological Research Database (GePaRD, claims data from approximately 20% of the German population), we conducted annual cross-sectional analyses to determine age-standardized prevalence of isotretinoin use between 2004 and 2019 among girls and women aged 13 to 49 years. In cohort analyses, we estimated the number of exposed pregnancies by assessing whether there was prescription supply overlapping the beginning of pregnancy (estimated supply was varied in sensitivity analyses) or a dispensation within the first 8 weeks of pregnancy. Data of live-born children classified as exposed in a critical period according to these criteria were reviewed to assess the presence of congenital malformations. The age-standardized prevalence of isotretinoin use per 1,000 girls and women increased from 1.20 (95% confidence interval [CI]: 1.16, 1.24) in 2004 to 1.96 (95% CI: 1.92, 2.01) in 2019. In the base case analysis, we identified 178 pregnancies exposed to isotretinoin, with the number per year doubling during the study period, and at least 45% of exposed pregnancies ended in an induced abortion. In sensitivity analyses, the number of exposed pregnancies ranged between 172 and 375. Among live-born children, 6 had major congenital malformations. The main limitation of this study was the lack of information on the prescribed dose, i.e., the supply had to be estimated based on the dispensed amount of isotretinoin.
Conclusions: Isotretinoin use among girls and women of childbearing age increased in Germany between 2004 and 2019, and there was a considerable number of pregnancies likely exposed to isotretinoin in a critical period. This highlights the importance of monitoring compliance with the existing risk minimization measures for isotretinoin in Germany.
Copyright: © 2024 Reinold et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist. JR, NW, KP, BK, and UH are working at an independent, non-profit research institute, the Leibniz Institute for Prevention Research and Epidemiology – BIPS. Unrelated to this study, BIPS occasionally conducts studies financed by the pharmaceutical industry. These are post-authorization safety studies (PASS) requested by health authorities. The design and conduct of these studies as well as the interpretation and publication are not influenced by the pharmaceutical industry. The study presented was not funded by the pharmaceutical industry.
Figures
References
-
- Marson JW, Baldwin HE. Isotretinoin update. Dermatol Rev. 2021;2(6):331–42. doi: 10.1002/der2.100 - DOI
-
- EMA. Updated measures for pregnancy prevention during retinoid use 2018. [cited 2023 Nov 14]. Available from: https://www.ema.europa.eu/en/documents/referral/retinoid-article-31-refe....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical