The forkhead transcription factor Foxj1 controls vertebrate olfactory cilia biogenesis and sensory neuron differentiation
- PMID: 38271330
- PMCID: PMC10810531
- DOI: 10.1371/journal.pbio.3002468
The forkhead transcription factor Foxj1 controls vertebrate olfactory cilia biogenesis and sensory neuron differentiation
Erratum in
-
Correction: The forkhead transcription factor Foxj1 controls vertebrate olfactory cilia biogenesis and sensory neuron differentiation.PLoS Biol. 2025 Jun 10;23(6):e3003229. doi: 10.1371/journal.pbio.3003229. eCollection 2025 Jun. PLoS Biol. 2025. PMID: 40493644 Free PMC article.
Abstract
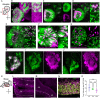
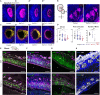
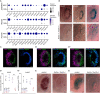
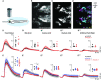
In vertebrates, olfactory receptors localize on multiple cilia elaborated on dendritic knobs of olfactory sensory neurons (OSNs). Although olfactory cilia dysfunction can cause anosmia, how their differentiation is programmed at the transcriptional level has remained largely unexplored. We discovered in zebrafish and mice that Foxj1, a forkhead domain-containing transcription factor traditionally linked with motile cilia biogenesis, is expressed in OSNs and required for olfactory epithelium (OE) formation. In keeping with the immotile nature of olfactory cilia, we observed that ciliary motility genes are repressed in zebrafish, mouse, and human OSNs. Strikingly, we also found that besides ciliogenesis, Foxj1 controls the differentiation of the OSNs themselves by regulating their cell type-specific gene expression, such as that of olfactory marker protein (omp) involved in odor-evoked signal transduction. In line with this, response to bile acids, odors detected by OMP-positive OSNs, was significantly diminished in foxj1 mutant zebrafish. Taken together, our findings establish how the canonical Foxj1-mediated motile ciliogenic transcriptional program has been repurposed for the biogenesis of immotile olfactory cilia, as well as for the development of the OSNs.
Copyright: © 2024 Rayamajhi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Evolutionarily ancient association of the FoxJ1 transcription factor with the motile ciliogenic program.PLoS Genet. 2012;8(11):e1003019. doi: 10.1371/journal.pgen.1003019. Epub 2012 Nov 8. PLoS Genet. 2012. PMID: 23144623 Free PMC article.
-
A Subset of Olfactory Sensory Neurons Express Forkhead Box J1-Driven eGFP.Chem Senses. 2019 Oct 26;44(9):663-671. doi: 10.1093/chemse/bjz060. Chem Senses. 2019. PMID: 31504289 Free PMC article.
-
Foxj1 transcription factors are master regulators of the motile ciliogenic program.Nat Genet. 2008 Dec;40(12):1445-53. doi: 10.1038/ng.263. Epub 2008 Nov 16. Nat Genet. 2008. PMID: 19011630
-
Maturation of the Olfactory Sensory Neuron and Its Cilia.Chem Senses. 2020 Dec 5;45(9):805-822. doi: 10.1093/chemse/bjaa070. Chem Senses. 2020. PMID: 33075817 Free PMC article. Review.
-
Switching on cilia: transcriptional networks regulating ciliogenesis.Development. 2014 Apr;141(7):1427-41. doi: 10.1242/dev.074666. Development. 2014. PMID: 24644260 Review.
Cited by
-
Ciliogenesis defects after neurulation impact brain development and neuronal activity in larval zebrafish.iScience. 2024 May 22;27(6):110078. doi: 10.1016/j.isci.2024.110078. eCollection 2024 Jun 21. iScience. 2024. PMID: 38868197 Free PMC article.
-
LRRC56 deletion causes primary ciliary dyskinesia in mice characterized by dynein arms defects.Biol Open. 2025 Feb 15;14(2):bio061846. doi: 10.1242/bio.061846. Epub 2025 Feb 5. Biol Open. 2025. PMID: 39912490 Free PMC article.
-
Correction: The forkhead transcription factor Foxj1 controls vertebrate olfactory cilia biogenesis and sensory neuron differentiation.PLoS Biol. 2025 Jun 10;23(6):e3003229. doi: 10.1371/journal.pbio.3003229. eCollection 2025 Jun. PLoS Biol. 2025. PMID: 40493644 Free PMC article.
-
Foxn3 is required to suppress aberrant ciliogenesis in nonphotoreceptor retinal neurons.Proc Natl Acad Sci U S A. 2025 Jul 22;122(29):e2500871122. doi: 10.1073/pnas.2500871122. Epub 2025 Jul 15. Proc Natl Acad Sci U S A. 2025. PMID: 40663603
-
Lagging Brain Gene Expression Patterns of Drosophila melanogaster Young Adult Males Confound Comparisons Between Sexes.Mol Neurobiol. 2025 Mar;62(3):2955-2972. doi: 10.1007/s12035-024-04427-7. Epub 2024 Aug 28. Mol Neurobiol. 2025. PMID: 39196495 Free PMC article.
References
-
- Ache BW, Young JM. Olfaction: Diverse Species, Conserved Principles. Neuron. 2005;48(3):417–430. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

