Effectiveness of Fludrocortisone Plus Hydrocortisone versus Hydrocortisone Alone in Septic Shock: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
- PMID: 38271488
- PMCID: PMC11146553
- DOI: 10.1164/rccm.202310-1785OC
Effectiveness of Fludrocortisone Plus Hydrocortisone versus Hydrocortisone Alone in Septic Shock: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Abstract
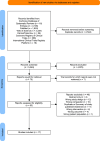
Rationale: The use of hydrocortisone in adult patients with septic shock is controversial, and the effectiveness of adding fludrocortisone to hydrocortisone remains uncertain. Objectives: To assess the comparative effectiveness and safety of fludrocortisone plus hydrocortisone, hydrocortisone alone, and placebo or usual care in adults with septic shock. Methods: A systematic review and a Bayesian network meta-analysis of peer-reviewed randomized trials were conducted. The primary outcome was all-cause mortality at last follow-up. Treatment effects are presented as relative risks (RRs) with 95% credible intervals (CrIs). Placebo or usual care was the reference treatment. Measurements and Main Results: Among 7,553 references, we included 17 trials (7,688 patients). All-cause mortality at last follow-up was lowest with fludrocortisone plus hydrocortisone (RR, 0.85; 95% CrI, 0.72-0.99; 98.3% probability of superiority, moderate-certainty evidence), followed by hydrocortisone alone (RR, 0.97; 95% CrI, 0.87-1.07; 73.1% probability of superiority, low-certainty evidence). The comparison of fludrocortisone plus hydrocortisone versus hydrocortisone alone was based primarily on indirect evidence (only two trials with direct evidence). Fludrocortisone plus hydrocortisone was associated with a 12% lower risk of all-cause mortality compared with hydrocortisone alone (RR, 0.88; 95% CrI, 0.74-1.03; 94.2% probability of superiority, moderate-certainty evidence). Conclusions: In adult patients with septic shock, fludrocortisone plus hydrocortisone was associated with lower risk of all-cause mortality at last follow-up than placebo and hydrocortisone alone. The scarcity of head-to-head trials comparing fludrocortisone plus hydrocortisone versus hydrocortisone alone led our network meta-analysis to rely primarily on indirect evidence for this comparison. Although we undertook several sensitivity analyses and assessments, these findings should be considered while also acknowledging the heterogeneity of included trials.
Keywords: adrenal cortex hormones; network meta-analysis; sepsis.
Figures


Comment in
-
Monotherapy or Combination Therapy for Septic Shock? A Debate on Steroids.Am J Respir Crit Care Med. 2024 May 15;209(10):1179-1180. doi: 10.1164/rccm.202401-0175ED. Am J Respir Crit Care Med. 2024. PMID: 38354067 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

