The Lung Microbiome Predicts Mortality and Response to Azithromycin in Lung Transplant Recipients with Chronic Rejection
- PMID: 38271553
- PMCID: PMC11146567
- DOI: 10.1164/rccm.202308-1326OC
The Lung Microbiome Predicts Mortality and Response to Azithromycin in Lung Transplant Recipients with Chronic Rejection
Abstract
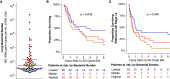
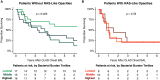
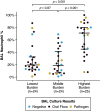
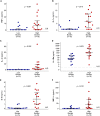
Rationale: Chronic lung allograft dysfunction (CLAD) is the leading cause of death after lung transplant, and azithromycin has variable efficacy in CLAD. The lung microbiome is a risk factor for developing CLAD, but the relationship between lung dysbiosis, pulmonary inflammation, and allograft dysfunction remains poorly understood. Whether lung microbiota predict outcomes or modify treatment response after CLAD is unknown. Objectives: To determine whether lung microbiota predict post-CLAD outcomes and clinical response to azithromycin. Methods: Retrospective cohort study using acellular BAL fluid prospectively collected from recipients of lung transplant within 90 days of CLAD onset. Lung microbiota were characterized using 16S rRNA gene sequencing and droplet digital PCR. In two additional cohorts, causal relationships of dysbiosis and inflammation were evaluated by comparing lung microbiota with CLAD-associated cytokines and measuring ex vivo P. aeruginosa growth in sterilized BAL fluid. Measurements and Main Results: Patients with higher bacterial burden had shorter post-CLAD survival, independent of CLAD phenotype, azithromycin treatment, and relevant covariates. Azithromycin treatment improved survival in patients with high bacterial burden but had negligible impact on patients with low or moderate burden. Lung bacterial burden was positively associated with CLAD-associated cytokines, and ex vivo growth of P. aeruginosa was augmented in BAL fluid from transplant recipients with CLAD. Conclusions: In recipients of lung transplants with chronic rejection, increased lung bacterial burden is an independent risk factor for mortality and predicts clinical response to azithromycin. Lung bacterial dysbiosis is associated with alveolar inflammation and may be promoted by underlying lung allograft dysfunction.
Keywords: chronic lung allograft dysfunction (CLAD); lung microbiome; lung transplantation.
Figures



Comment in
-
Definitions of Dysbiosis in Chronic Lung Allograft Dysfunction and High Bacterial Biomass.Am J Respir Crit Care Med. 2024 Jun 1;209(11):1296-1298. doi: 10.1164/rccm.202402-0451ED. Am J Respir Crit Care Med. 2024. PMID: 38536158 Free PMC article. No abstract available.
References
-
- Titman A, Rogers CA, Bonser RS, Banner NR, Sharples LD. Disease-specific survival benefit of lung transplantation in adults: a national cohort study. Am J Transplant . 2009;9:1640–1649. - PubMed
-
- Chambers DC, Perch M, Zuckermann A, Cherikh WS, Harhay MO, Hayes D, Jr, et al. International Society for Heart and Lung Transplantation The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report—2021; focus on recipient characteristics. J Heart Lung Transplant . 2021;40:1060–1072. - PMC - PubMed
-
- Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D, Jr, Kucheryavaya AY, et al. International Society for Heart and Lung Transplantation The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant . 2018;37:1155–1168. - PubMed
-
- Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD. Long-term kidney transplant graft survival—making progress when most needed. Am J Transplant . 2021;21:2824–2832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

