Plasmin-cleaved von Willebrand factor as a biomarker for microvascular thrombosis
- PMID: 38271661
- PMCID: PMC11143499
- DOI: 10.1182/blood.2023021265
Plasmin-cleaved von Willebrand factor as a biomarker for microvascular thrombosis
Abstract
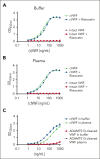
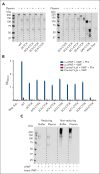
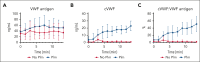
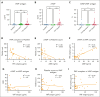
von Willebrand factor (VWF) is an essential contributor to microvascular thrombosis. Physiological cleavage by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) limits its prothrombotic properties, explaining why ADAMTS13 deficiency leads to attacks of microthrombosis in patients with thrombotic thrombocytopenic purpura (TTP). We previously reported that plasminogen activation takes place during TTP attacks in these patients. Furthermore, stimulation of plasminogen activation attenuates pathogenesis in preclinical TTP models in vivo. This suggests that plasmin is an endogenous regulator of VWF thrombogenicity, in particular when ADAMTS13 falls short to prevent microvascular occlusions. VWF cleavage by plasmin is biochemically distinct from cleavage by ADAMTS13. We hypothesized that plasmin-cleaved VWF (cVWF) holds value as a biomarker of microvascular thrombosis. Here, we describe the development of a variable domain of heavy-chain-only antibody (VHH)-based bioassay that can distinguish cVWF from intact and ADAMTS13-cleaved VWF in plasma. We validate this assay by tracking cVWF release during degradation of microthombi in vitro. We demonstrate that endogenous cVWF formation takes place in patients with TTP during acute attacks of thrombotic microangiopathy but not in those in remission. Finally, we show that therapeutic plasminogen activation in a mouse model of TTP amplifies cVWF formation, which is accompanied by VWF clearance. Our combined findings indicate that cVWF is released from microthrombi in the context of microvascular occlusion.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.d.M. is a cofounder of TargED Biopharmaceuticals BV, a biotech spinout company of University Medical Center Utrecht, and participates in revenue sharing as inventor through the commercialization arm of the University Medical Center Utrecht. The results discussed in this article form part of a pending patent application. The remaining authors declare no competing financial interests.
Figures



Comment in
-
A "backup plan" for ADAMTS13 deficiency in TTP.Blood. 2024 May 16;143(20):2021-2023. doi: 10.1182/blood.2024024065. Blood. 2024. PMID: 38753355 No abstract available.
References
-
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. J Am Med Assoc. 2014;311(11):1117–1124. - PubMed
-
- Favresse J, Lippi G, Roy PM, et al. D-dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548–577. - PubMed
-
- Tersteeg C, De Maat S, De Meyer SF, et al. Plasmin cleavage of von willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation. 2014;129(12):1320–1331. - PubMed
-
- Tersteeg C, Joly BS, Gils A, et al. Amplified endogenous plasmin activity resolves acute thrombotic thrombocytopenic purpura in mice. J Thromb Haemost. 2017;15(12):2432–2442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

