Neuropsychological Assessments of Cognitive Impairment in Major Depressive Disorder: A Systematic Review and Meta-Analysis with Meta-Regression
- PMID: 38272009
- PMCID: PMC10880806
- DOI: 10.1159/000535665
Neuropsychological Assessments of Cognitive Impairment in Major Depressive Disorder: A Systematic Review and Meta-Analysis with Meta-Regression
Abstract
Introduction: Cognitive dysfunction or deficits are common in patients with major depressive disorder (MDD). The current study systematically reviews and meta-analyzes multiple domains of cognitive impairment in patients with MDD.
Methods: PubMed/MEDLINE, PsycINFO, Cochrane Library, Embase, Web of Science, and Google Scholar were searched from inception through May 17, 2023, with no language limits. Studies with the following inclusion criteria were included: (1) patients with a diagnosis of MDD using standardized diagnostic criteria; (2) healthy controls (i.e., those without MDD); (3) neuropsychological assessments of cognitive impairment using Cambridge Neuropsychological Test Automated Battery (CANTAB); and (4) reports of sufficient data to quantify standardized effect sizes. Hedges' g standardized mean differences (SMDs) with corresponding 95% confidence intervals (CIs) were used to quantify effect sizes of cognitive impairments in MDD. SMDs were estimated using a fixed- or random-effects models.
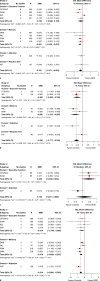
Results: Overall, 33 studies consisting of 2,596 subjects (n = 1,337 for patients with MDD and n = 1,259 for healthy controls) were included. Patients with MDD, when compared to healthy controls, had moderate cognitive deficits (SMD, -0.39 [95% CI, -0.47 to -0.31]). In our subgroup analyses, patients with treatment-resistant depression (SMD, -0.56 [95% CI, -0.78 to -0.34]) and older adults with MDD (SMD, -0.51 [95% CI, -0.66 to -0.36]) had greater cognitive deficits than healthy controls. The effect size was small among unmedicated patients with MDD (SMD, -0.19 [95% CI, -0.37 to -0.00]), and we did not find any statistical difference among children. Cognitive deficits were consistently found in all domains, except the reaction time. No publication bias was reported.
Conclusion: Because cognitive impairment in MDD can persist in remission or increase the risk of major neurodegenerative disorders, remediation of cognitive impairment in addition to alleviation of depressive symptoms should be an important goal when treating patients with MDD.
Keywords: Cambridge Neuropsychological Test Automated Battery; Cognition; Cognitive impairment; Depression; Major depressive disorder.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Each author completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and had none directly related to this manuscript. Rhee was supported in part by the National Institute on Aging (NIA) (#R21AG070666; R21AG078972), the National Institute of Mental Health (#R21MH117438), the National Institute on Drug Abuse (#R21DA057540), and the Institute for Collaboration on Health, Intervention, and Policy (InCHIP) of the University of Connecticut. Dr. Rhee serves as a review committee member for Patient-Centered Outcomes Research Institute (PCORI) and Substance Abuse and Mental Health Services Administration (SAMHSA) and has received honoraria payments from PCORI and SAMHSA. Dr. Rhee has also served as a stakeholder/consultant for PCORI and received consulting fees from PCORI. Dr. Rhee serves as an advisory committee member for International Alliance of Mental Health Research Funders (IAMHRF). Dr. Rhee is currently a co-editor-in-chief of Mental Health Science and has received honorarium payments annually from the publisher, John Wiley & Sons, Inc. Shim, Kaster, D’Andrea, and Steffens reported none. Manning has received research grant funding from the National Institute of Mental Health (#K21MH118420) and the Alzheimer’s Association. He serves as a consultant for Brandywine Assisted Living. Tennen was supported in part by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (#P50AA027055 and #T32AA00729) and by the National Institute of Dental and Craniofacial Research (NIDCR) (#U01DE028520). He serves as a consultant to Yale University School of Medicine (NIAAA #R01AA026844) for which he receives a consulting fee. Dr. Tennen is the editor of the Journal of Personality and receives honorarium payments annually from the publisher, John Wiley & Sons, Inc. Forester has received research grant funding from Biogen, Eisai, Rogers Family Foundation, Spier Family Foundation, and the NIH. He also serves in the Pharmacy and Therapeutics Committee for CVS Health and is a consultant for Patina Health and Rippl Care. Nierenberg is supported by grants from the Patient-Centered Outcomes Research Institute (PaCR-2017C2-8169, XPPRN-1512-33786, XPPRN-1512-33786), the Thomas P. Hackett, MD Endowed Chair in Psychiatry at MGH, and the Dauten Family Center for Bipolar Treatment Innovation. Nierenberg is a consultant for Abbott Laboratories, Alkermes, American Psychiatric Association, Appliance Computing Inc., Mindsite, Basilea, BrainCells Inc., Brandeis University, Bristol-Myers Squibb, Clintara, Corcept, Dey Pharmaceuticals, Dainippon Sumitomo (now Sunovion), Eli Lilly, Epiq, Mylan, Forest, Genaissance, Genentech, GlaxoSmithKline, Hoffmann-La Roche, Infomedics, Intra-Cellular Therapies, Lundbeck, Janssen Pharmaceutical, Jazz Pharmaceuticals, MedAvante, Merck, Methylation Sciences, Naurex, NeuroRx, Novartis, Otsuka, Pamlabs, Parexel, Pfizer, PGx Health, Ridge Diagnostics Shire, Schering-Plough, Somerset, Sunovion, Takeda Pharmaceuticals, Targacept, and Teva; consulted through the Massachusetts General Hospital Clinical Trials Network and Institute for AstraZeneca, BrainCells Inc., Dainippon Sumitomo/Sepracor, Johnson and Johnson, LaboPharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals; receives grant or research support from American Foundation for Suicide Prevention, Agency for Healthcare Research and Quality, Brain and Behavior Research Foundation, Bristol-Myers Squibb, Cederroth, Cephalon, Cyberonics, Elan, Eli Lilly, Forest, GlaxoSmithKline, Janssen Pharmaceutical, Intra-Cellular Therapies, Lichtwer Pharma, Marriott Foundation, Mylan, National Institute of Mental Health, Pamlabs, Patient-Centered Outcomes Research Institute, Pfizer, Shire, Stanley Foundation, Takeda, and Wyeth-Ayerst; honoraria include Belvoir Publishing, University of Texas Southwestern Dallas, Brandeis University, Bristol-Myers Squibb, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Baystate Medical Center, Columbia University, Controlled Risk Insurance Company, Dartmouth Medical School, Health New England, Harold Grinspoon Charitable Foundation, Imedex, Israel Society for Biological Psychiatry, Johns Hopkins University, MJ Consulting, New York State, Medscape, MBL Communications Inc., Massachusetts General Hospital Psychiatry Academy, National Association of Continuing Education, Physicians Postgraduate Press, State University of New York Buffalo, University of Wisconsin, University of Pisa, University of Michigan, University of Miami, University of Wisconsin at Madison, World Congress of Brain Behavior and Emotion, American Professional Society of ADHD and Related Disorders, International Society for Bipolar Disorder, SciMed, Slack Publishing, Wolters Kluwer Publishing, the American Society for Clinical Psychopharmacology (formerly NCDEU), Rush Medical College, Yale University School of Medicine, National Nuclear Data Center, Nova Southeastern University, National Alliance on Mental Illness, Institute of Medicine, Continued Medical Education Institute, and the International Society for CNS Clinical Trials and Methodology; is currently or was formerly on the advisory boards of Appliance Computing Inc., BrainCells Inc., Eli Lilly, Genentech, Johnson and Johnson, Takeda/Lundbeck, Targacept, and Infomedics; owns stock options in Appliance Computing Inc., BrainCells Inc., and MedAvante; has copyrights to the Clinical Positive Affect Scale and the Massachusetts General Hospital Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the Massachusetts General Hospital Clinical Trials Network and Institute.McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Mitsubishi, Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, Atai Life Sciences. Dr. Roger McIntyre is a CEO of Braxia Scientific Corp.
Figures


Similar articles
-
Cognitive function following a major depressive episode: a systematic review and meta-analysis.Lancet Psychiatry. 2019 Oct;6(10):851-861. doi: 10.1016/S2215-0366(19)30291-3. Epub 2019 Aug 15. Lancet Psychiatry. 2019. PMID: 31422920
-
Cognitive Performance in First-Degree Relatives of Individuals With vs Without Major Depressive Disorder: A Meta-analysis.JAMA Psychiatry. 2019 Mar 1;76(3):297-305. doi: 10.1001/jamapsychiatry.2018.3672. JAMA Psychiatry. 2019. PMID: 30586133 Free PMC article.
-
Cognitive rehabilitation for improving cognitive functions and reducing the severity of depressive symptoms in adult patients with Major Depressive Disorder: a systematic review and meta-analysis of randomized controlled clinical trials.BMC Psychiatry. 2023 Jan 27;23(1):77. doi: 10.1186/s12888-023-04554-w. BMC Psychiatry. 2023. PMID: 36707847 Free PMC article.
-
Long-term cognitive effects of electroconvulsive therapy in major depressive disorder: A systematic review and meta-analysis.Psychiatry Res. 2024 Jan;331:115611. doi: 10.1016/j.psychres.2023.115611. Epub 2023 Dec 1. Psychiatry Res. 2024. PMID: 38101070
-
Deployment of personnel to military operations: impact on mental health and social functioning.Campbell Syst Rev. 2018 Jun 1;14(1):1-127. doi: 10.4073/csr.2018.6. eCollection 2018. Campbell Syst Rev. 2018. PMID: 37131363 Free PMC article.
Cited by
-
Cognitive Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Aetiology and Potential Treatments.Int J Mol Sci. 2025 Feb 22;26(5):1896. doi: 10.3390/ijms26051896. Int J Mol Sci. 2025. PMID: 40076522 Free PMC article. Review.
-
Identification of risk- and preventive factors predicting child maltreatment in pregnant women with psychosocial problems.Front Psychiatry. 2025 May 21;16:1552740. doi: 10.3389/fpsyt.2025.1552740. eCollection 2025. Front Psychiatry. 2025. PMID: 40469377 Free PMC article.
-
Improvement of persistent impairments in executive functions and attention following electroconvulsive therapy in a case control longitudinal follow up study.BMC Psychiatry. 2024 Nov 20;24(1):832. doi: 10.1186/s12888-024-06270-5. BMC Psychiatry. 2024. PMID: 39567961 Free PMC article.
-
Parsing the heterogeneity of depression: a data-driven subgroup derived from cognitive function.Front Psychiatry. 2025 Jan 30;16:1537331. doi: 10.3389/fpsyt.2025.1537331. eCollection 2025. Front Psychiatry. 2025. PMID: 39950172 Free PMC article.
-
Depression reduces cognitive function partly through effects on BMI and hypertension: a large observational study and Mendelian randomization analysis.BMC Psychiatry. 2025 Apr 17;25(1):393. doi: 10.1186/s12888-025-06846-9. BMC Psychiatry. 2025. PMID: 40247239 Free PMC article.
References
-
- World Health Organization . Depressive disorder (depression). 2023.
-
- McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. . Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–27. - PubMed
-
- Rhee TG, Steffens DC. Major depressive disorder and impaired health-related quality of life among US older adults. Int J Geriatr Psychiatry. 2020;35(10):1189–97. - PubMed

