Oncogenic Fatty Acid Metabolism Rewires Energy Supply Chain in Gastric Carcinogenesis
- PMID: 38272100
- PMCID: PMC11040571
- DOI: 10.1053/j.gastro.2024.01.027
Oncogenic Fatty Acid Metabolism Rewires Energy Supply Chain in Gastric Carcinogenesis
Abstract
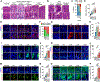
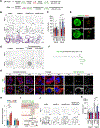
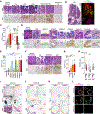
Background & aims: Gastric carcinogenesis develops within a sequential carcinogenic cascade from precancerous metaplasia to dysplasia and adenocarcinoma, and oncogenic gene activation can drive the process. Metabolic reprogramming is considered a key mechanism for cancer cell growth and proliferation. However, how metabolic changes contribute to the progression of metaplasia to dysplasia remains unclear. We have examined metabolic dynamics during gastric carcinogenesis using a novel mouse model that induces Kras activation in zymogen-secreting chief cells.
Methods: We generated a Gif-rtTA;TetO-Cre;KrasG12D (GCK) mouse model that continuously induces active Kras expression in chief cells after doxycycline treatment. Histologic examination and imaging mass spectrometry were performed in the GCK mouse stomachs at 2 to 14 weeks after doxycycline treatment. Mouse and human gastric organoids were used for metabolic enzyme inhibitor treatment. The GCK mice were treated with a stearoyl- coenzyme A desaturase (SCD) inhibitor to inhibit the fatty acid desaturation. Tissue microarrays were used to assess the SCD expression in human gastrointestinal cancers.
Results: The GCK mice developed metaplasia and high-grade dysplasia within 4 months. Metabolic reprogramming from glycolysis to fatty acid metabolism occurred during metaplasia progression to dysplasia. Altered fatty acid desaturation through SCD produces a novel eicosenoic acid, which fuels dysplastic cell hyperproliferation and survival. The SCD inhibitor killed both mouse and human dysplastic organoids and selectively targeted dysplastic cells in vivo. SCD was up-regulated during carcinogenesis in human gastrointestinal cancers.
Conclusions: Active Kras expression only in gastric chief cells drives the full spectrum of gastric carcinogenesis. Also, oncogenic metabolic rewiring is an essential adaptation for high-energy demand in dysplastic cells.
Keywords: Carcinogenesis; Fatty Acid Metabolism; Imaging Mass Spectrometry; Kras; Stearoyl-CoA Desaturase.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures



References
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022;12:31–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

