Emerging roles of O-GlcNAcylation in protein trafficking and secretion
- PMID: 38272225
- PMCID: PMC10907171
- DOI: 10.1016/j.jbc.2024.105677
Emerging roles of O-GlcNAcylation in protein trafficking and secretion
Abstract
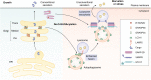
The emerging roles of O-GlcNAcylation, a distinctive post-translational modification, are increasingly recognized for their involvement in the intricate processes of protein trafficking and secretion. This modification exerts its influence on both conventional and unconventional secretory pathways. Under healthy and stress conditions, such as during diseases, it orchestrates the transport of proteins within cells, ensuring timely delivery to their intended destinations. O-GlcNAcylation occurs on key factors, like coat protein complexes (COPI and COPII), clathrin, SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors), and GRASP55 (Golgi reassembly stacking protein of 55 kDa) that control vesicle budding and fusion in anterograde and retrograde trafficking and unconventional secretion. The understanding of O-GlcNAcylation offers valuable insights into its critical functions in cellular physiology and the progression of diseases, including neurodegeneration, cancer, and metabolic disorders. In this review, we summarize and discuss the latest findings elucidating the involvement of O-GlcNAc in protein trafficking and its significance in various human disorders.
Keywords: COPI; COPII; GRASP55; O-GlcNAcylation; autophagy; cancer; clathrin; conventional secretion; exosome; metabolic disease; neurodegeneration; protein trafficking; unconventional secretion.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Cook K.M., Hogg P.J. Post-translational control of protein function by disulfide bond cleavage. Antioxid. Redox Signal. 2013;18:1987–2015. - PubMed
-
- Schjoldager K.T., Narimatsu Y., Joshi H.J., Clausen H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020;21:729–749. - PubMed
-
- Ohtsubo K., Marth J.D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

