Elongation factor 1A1 regulates metabolic substrate preference in mammalian cells
- PMID: 38272231
- PMCID: PMC10891338
- DOI: 10.1016/j.jbc.2024.105684
Elongation factor 1A1 regulates metabolic substrate preference in mammalian cells
Abstract
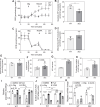
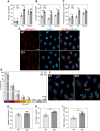
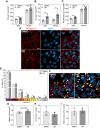
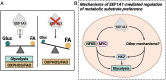
Eukaryotic elongation factor 1A1 (EEF1A1) is canonically involved in protein synthesis but also has noncanonical functions in diverse cellular processes. Previously, we identified EEF1A1 as a mediator of lipotoxicity and demonstrated that chemical inhibition of EEF1A1 activity reduced mouse liver lipid accumulation. These findings suggested a link between EEF1A1 and metabolism. Therefore, we investigated its role in regulating metabolic substrate preference. EEF1A1-deficient Chinese hamster ovary (2E2) cells displayed reduced media lactate accumulation. These effects were also observed with EEF1A1 knockdown in human hepatocyte-like HepG2 cells and in WT Chinese hamster ovary and HepG2 cells treated with selective EEF1A inhibitors, didemnin B, or plitidepsin. Extracellular flux analyses revealed decreased glycolytic ATP production and increased mitochondrial-to-glycolytic ATP production ratio in 2E2 cells, suggesting a more oxidative metabolic phenotype. Correspondingly, fatty acid oxidation was increased in 2E2 cells. Both 2E2 cells and HepG2 cells treated with didemnin B exhibited increased neutral lipid content, which may be required to support elevated oxidative metabolism. RNA-seq revealed a >90-fold downregulation of a rate-limiting glycolytic enzyme, hexokinase 2, which we confirmed through immunoblotting and enzyme activity assays. Pathway enrichment analysis identified downregulations in TNFA signaling via NFKB and MYC targets. Correspondingly, nuclear abundances of RELB and MYC were reduced in 2E2 cells. Thus, EEF1A1 deficiency may perturb glycolysis by limiting NFKB- and MYC-mediated gene expression, leading to decreased hexokinase expression and activity. This is the first evidence of a role for a translation elongation factor, EEF1A1, in regulating metabolic substrate utilization in mammalian cells.
Keywords: glucose; glycolysis; lipid; lipid metabolism; translation elongation factor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Man O., Pilpel Y. Differential translation efficiency of orthologous genes is involved in phenotypic divergence of yeast species. Nat. Genet. 2007;39:415–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

