Novel Benzofuran Derivatives Induce Monoamine Release and Substitute for the Discriminative Stimulus Effects of 3,4-Methylenedioxymethamphetamine
- PMID: 38272669
- PMCID: PMC11413916
- DOI: 10.1124/jpet.123.001837
Novel Benzofuran Derivatives Induce Monoamine Release and Substitute for the Discriminative Stimulus Effects of 3,4-Methylenedioxymethamphetamine
Abstract
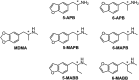
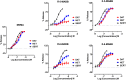
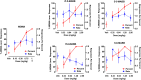
3,4-Methylenedioxymethamphetamine (MDMA) has shown efficacy as a medication adjunct for treating post-traumatic stress disorder (PTSD). However, MDMA is also used in nonmedical contexts that pose risk for cardiovascular and neurologic complications. It is well established that MDMA exerts its effects by stimulating transporter-mediated release of the monoamines 5-hydroxytryptamine (5-HT), norepinephrine, and dopamine. Current research efforts are aimed at developing MDMA-like monoamine releasers with better efficacy and safety profiles. To this end, we investigated neurochemical and behavioral effects of novel analogs of the designer drug 5-(2-methylaminopropyl)benzofuran (5-MAPB). We used in vitro transporter assays in rat brain synaptosomes to examine transmitter uptake inhibition and releasing properties for enantiomers of 5-(2-methylaminobutyl)benzofuran (5-MABB) and 6-(2-methylaminobutyl)benzofuran (6-MABB) compared with MDMA. We then tested these same compounds in male Sprague-Dawley rats trained to discriminate MDMA (1.5 mg/kg) from saline. In vitro results revealed that S isomers of 5- and 6-MABB are efficacious releasing agents at transporters for 5-HT (SERT), norepinephrine (NET), and dopamine (DAT). By contrast, R isomers are efficacious releasers at SERT and partial releasers at NET but lack releasing activity at DAT. In vivo results showed that all compounds produce dose-dependent increases in MDMA-lever responding and full substitution at the highest dose tested. The diminished NET and DAT releasing activities for R isomers of 5- and 6-MABB are associated with reduced potency for inducing behavioral effects. Collectively, these findings indicate that the aminoalkyl benzofuran scaffold may be a viable template for developing compounds with MDMA-like properties. SIGNIFICANCE STATEMENT: Despite the clinical utility of 3,4-methylenedioxymethamphetamine (MDMA), the drug is associated with certain cardiovascular risks and metabolic side effects. Developing a therapeutic alternative with MDMA-like monoamine releasing activity is of interest. Our in vitro and in vivo findings indicate that the aminoalkyl benzofuran scaffold may be useful for developing compounds with MDMA-like properties.
U.S. Government work not protected by U.S. copyright.
Figures

References
-
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB (1995) Assessment of the discriminative stimulus effects of the optical isomers of Ecstasy (3,4-methylenedioxymethamphetamine; MDMA). Behav Pharmacol 6:263–275. - PubMed
-
- Baker LE, Taylor MM (1997) Assessment of the MDA and MDMA optical isomers in a stimulant-hallucinogen discrimination. Pharmacol Biochem Behav 57:737–748. - PubMed
-
- Boeckel MG, Wagner A, Grassi-Oliveira R (2017) The effects of intimate partner violence exposure on the maternal bond and PTSD symptoms of children. J Interpers Violence 32:1127–1142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
