Mixed Cluster Ions of Magnesium and C60
- PMID: 38272839
- PMCID: PMC10860146
- DOI: 10.1021/acs.jpca.3c06902
Mixed Cluster Ions of Magnesium and C60
Abstract
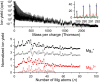
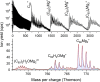
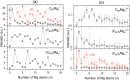
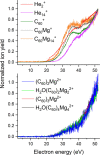
Magnesium clusters exhibit a pronounced nonmetal-to-metal transition, and the neutral dimer is exceptionally weakly bound. In the present study, we formed pristine Mgnz+ (n = 1-100, z = 1-3) clusters and mixed (C60)mMgnz+ clusters (m = 1-7, z = 1, 2) upon electron irradiation of neutral helium nanodroplets doped with magnesium or a combination of C60 and magnesium. The mass spectra obtained for pristine magnesium cluster ions exhibit anomalies, consistent with previous reports in the literature. The anomalies observed for C60Mgn+ strongly suggest that Mg atoms tend to wet the surface of the single fullerene positioning itself above the center of a pentagonal or hexagonal face, while, for (C60)mMgnz+, the preference for Mg to position itself within the dimples formed by fullerene cages becomes apparent. Besides doubly charged cluster ions, with the smallest member Mg22+, we also observed the formation of triply charged ions Mgn3+ with n > 24. The ion efficiency curves of singly and multiply charged ions exhibit pronounced differences compared to singly charged ions at higher electron energies. These findings indicate that sequential Penning ionization is essential in the formation of doubly and triply charged ions inside doped helium nanodroplets.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- German E.; Hou G. L.; Vanbuel J.; Bakker J. M.; Alonso J. A.; Janssens E.; Lopez M. J. Infrared spectra and structures of C60Rhn+ complexes. Carbon 2022, 197, 535–543. 10.1016/j.carbon.2022.07.002. - DOI
-
- Bashiri S.; Vessally E.; Bekhradnia A.; Hosseinian A.; Edjlali L. Utility of extrinsic [60] fullerenes as work function type sensors for amphetamine drug detection: DFT studies. Vacuum 2017, 136, 156–162. 10.1016/j.vacuum.2016.12.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

