RORα-GABP-TFAM axis alleviates myosteatosis with fatty atrophy through reinforcement of mitochondrial capacity
- PMID: 38272857
- PMCID: PMC10995264
- DOI: 10.1002/jcsm.13432
RORα-GABP-TFAM axis alleviates myosteatosis with fatty atrophy through reinforcement of mitochondrial capacity
Abstract
Background: Fat infiltration in muscle, called 'myosteatosis', precedes muscle atrophy, which subsequently results in sarcopenia. Myosteatosis is frequently observed in patients with nonalcoholic fatty liver disease (NAFLD). We have previously reported that retinoic acid receptor-related orphan receptor-α (RORα) regulates mitochondrial dynamics and mitophagy in hepatocytes, resulting in an alleviation of NAFLD. In this study, we aimed to investigate the role of RORα in skeletal muscle and to understand molecular mechanisms by which RORα controls mitochondrial capacity, using an NAFLD-associated myosteatosis mouse model.
Methods: To establish a myosteatosis model, 7-week-old C57BL/6N mice were fed with high-fat diet (HFD). After 15 weeks of diet feeding, an adeno-associated virus vector encoding RORα (AAV-RORα) was injected to gastrocnemius (GA) muscles, or after 7 weeks of HFD feeding, JC1-40, an RORα agonistic ligand, was administered daily at a dose of 5 mg/kg/day by oral gavage for 5 weeks. Histological, biochemical and molecular analyses in various in vivo and in vitro experiments were performed.
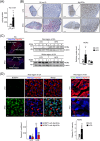
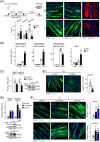
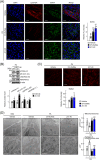
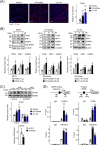
Results: First, the number of oxidative MyHC2a fibres with intensive lipid infiltration increased by 3.8-fold in the red region of the GA of mice with myosteatosis (P < 0.001). RORα was expressed around MyHC2a fibres, and its level increased by 2.7-fold after HFD feeding (P < 0.01). Second, treatment of RORα ligands in C2C12 myoblasts, such as cholesterol sulfate and JC1-40, enhanced the number of oxidative fibres stained for MyHC1 and MyHC2a by two-fold to four-fold (P < 0.01), while it reduced the lipid levels in MyHC2a fibres by 20-50% (P < 0.001) in the presence of palmitic acids. Third, mitochondrial membrane potential (P < 0.01) and total area of mitochondria (P < 0.01) were enhanced by treatment of these ligands. Chromatin immunoprecipitation analysis showed that RORα bound the promoter of GA-binding protein α subunit gene that led to activation of mitochondrial transcription factor A (TFAM) in C2C12 myoblasts (P < 0.05). Finally, intramuscular transduction of AAV-RORα alleviated the HFD-induced myosteatosis with fatty atrophy; lipid contents in MyHC2a fibres decreased by 48% (P < 0.001), whereas the number of MyHC2b fibre increased by 22% (P < 0.001). Also, administration of JC1-40 improved the signs of myosteatosis in that it decreased the level of adipose differentiation-related protein (P < 0.01) but increased mitochondrial proteins such as cytochrome c oxidase 4 and TFAM in GA muscle (P < 0.01).
Conclusions: RORα plays a versatile role in regulating the quantity of mitochondria and the oxidative capacity, ultimately leading to an improvement in myosteatosis symptoms.
Keywords: NAFLD; RORα; fatty atrophy; mitochondrial biogenesis; myosteatosis.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

