Exposure-response relationship of cabozantinib in patients with metastatic renal cell carcinoma treated in routine care
- PMID: 38272963
- PMCID: PMC10950854
- DOI: 10.1038/s41416-024-02585-y
Exposure-response relationship of cabozantinib in patients with metastatic renal cell carcinoma treated in routine care
Abstract
Background: Interindividual pharmacokinetic variability may influence the clinical benefit or toxicity of cabozantinib in metastatic renal cell carcinoma (mRCC). We aimed to investigate the exposure-toxicity and exposure-response relationship of cabozantinib in unselected mRCC patients treated in routine care.
Methods: This ambispective multicenter study enrolled consecutive patients receiving cabozantinib in monotherapy. Steady-state trough concentration (Cmin,ss) within the first 3 months after treatment initiation was used for the PK/PD analysis with dose-limiting toxicity (DLT) and survival outcomes. Logistic regression and Cox proportional-hazards models were used to identify the risk factors of DLT and inefficacy in patients, respectively.
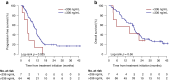
Results: Seventy-eight mRCC patients were eligible for the statistical analysis. Fifty-two patients (67%) experienced DLT with a median onset of 2.1 months (95%CI 0.7-8.2). In multivariate analysis, Cmin,ss was identified as an independent risk factor of DLT (OR 1.46, 95%CI [1.04-2.04]; p = 0.029). PFS and OS were not statistically associated with the starting dose (p = 0.81 and p = 0.98, respectively). In the multivariate analysis of PFS, Cmin, ss > 336 ng/mL resulted in a hazard ratio of 0.28 (95%CI, 0.10-0.77, p = 0.014). By contrast, Cmin, ss > 336 ng/mL was not statistically associated with longer OS.
Conclusion: Early plasma drug monitoring may be useful to optimise cabozantinib treatment in mRCC patients treated in monotherapy, especially in frail patients starting at a lower than standard dose.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
BB has received consulting and speaking fees (Bristol Myers Squibb, Clovis Oncology, Janssen, Pierre Fabre, Pfizer and Promise). SO has received honoraria (Sanofi, Pfizer, Bristol Myers Squibb, Eisai, Merck, Novartis, Ipsen, Astellas, Janssen and Bayer), travel and accommodation expenses (Sanofi, Pfizer, Bristol Myers Squibb, Eisai, Merck, Novartis, Ipsen, Astellas, Janssen and Bayer) and research grants (Sanofi, Astra Zeneca, Pfizer, Novartis, Janssen, Bayer and Roche). YV has received honoraria (Bristol Myers Squibb, Merck, Ipsen, Pfizer and Eisai) and research grants (Bristol Myers Squibb and Ipsen). CJ has received honoraria (Ipsen, Astellas, Bayer and Janssen). RF has received honoraria (Bayer, Astellas, Janssen, Ipsen, Merck, Bristol Myers Squibb, Pfizer and Merck). AP has received speaking fees (Bristol Myers Squibb and Pierre Fabre). LM has received honoraria (Sanofi, Astellas, Janssen, MSD, Bristol Myers Squibb, Ipsen, Astra Zeneca and Merck, Novartis) and travel and accommodation expenses (Sanofi, Astellas, Janssen, Bristol Myers, Ipsen, Astra Zeneca, Pfizer, MSD). LA has received honoraria (Novartis, Astellas, Janssen, MSD, Bristol Myers Squibb, Ipsen, Eisai, Pfizer and Merck, Roche) and travel and accommodation expenses (Bristol Myers Squibb, Ipsen, Pfizer, MSD). OH has received honoraria (Sanofi, Bayer, MSD, Bristol Myers Squibb, Ipsen, Pfizer, Eisai, Janssen, Astra Zeneca and Merck). XD has received consulting honoraria (Inflectis Biosciences, MedDay Pharmaceuticals, MAPREG and Merck). JM has received consulting honoraria (Daiichi Sankyo, Gilead, Lilly Eli, MSD and Pfizer) and travel and accommodation expenses (Lilly Eli, Gilead and Seattle Genetics).AXV, AJ, FP, DC, FLL, FT, MT, TP, CT, JC, EC, MV and FG declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

