Dihydroartemisinin-piperaquine effectiveness for seasonal malaria chemoprevention in settings with extended seasonal malaria transmission in Tanzania
- PMID: 38273019
- PMCID: PMC10810795
- DOI: 10.1038/s41598-024-52706-z
Dihydroartemisinin-piperaquine effectiveness for seasonal malaria chemoprevention in settings with extended seasonal malaria transmission in Tanzania
Abstract
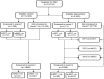
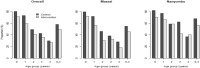
Effectiveness of dihydroartemisinin-piperaquine (DP) as seasonal malaria chemoprevention (SMC) was assessed in Nanyumbu and Masasi Districts. Between March and June 2021, children aged 3-59 months were enrolled in a cluster randomized study. Children in the intervention clusters received a monthly, 3-days course of DP for three consecutive months regardless of malaria infection status, and those in the control clusters received no intervention. Malaria infection was assessed at before the first-round and at 7 weeks after the third-round of DP in both arms. Malaria prevalence after the third-round of DP administration was the primary outcome. Chi-square tests and logistic regression model were used to compare proportions and adjust for explanatory variables. Before the intervention, malaria prevalence was 13.7% (161/1171) and 18.2% (212/1169) in the intervention and control clusters, respectively, p < 004. Malaria prevalence declined to 5.8% (60/1036) in the intervention clusters after three rounds of DP, and in the control clusters it declined to 9.3% (97/1048), p = 0.003. Unadjusted and adjusted prevalence ratios between the intervention and control arms were 0.42 (95%CI 0.32-0.55, p < 0.001) and 0.77 (95%CI 0.53-1.13, p = 0.189), respectively. SMC using DP was effective for control of malaria in the two Districts.Trial registration: NCT05874869, https://clinicaltrials.gov/ 25/05/2023.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. World Malaria Report 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240015791 (2020).
-
- WHO. World Malaria Report 2015. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/200018 (2015).
-
- WHO. World Malaria Report 2017. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789241565523 (2017).
-
- WHO. World Malaria Report 2010. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/44451 (2010).
-
- WHO. Intermittent Preventive Treatment of malaria in pregnancy using Sulfadoxine- Pyrimethamine (IPTp-SP). World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/WHO-HTM-GMP-2014.4 (2012).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

