Mitochondrial genomes revisited: why do different lineages retain different genes?
- PMID: 38273274
- PMCID: PMC10809612
- DOI: 10.1186/s12915-024-01824-1
Mitochondrial genomes revisited: why do different lineages retain different genes?
Abstract
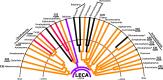
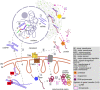
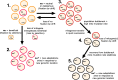
The mitochondria contain their own genome derived from an alphaproteobacterial endosymbiont. From thousands of protein-coding genes originally encoded by their ancestor, only between 1 and about 70 are encoded on extant mitochondrial genomes (mitogenomes). Thanks to a dramatically increasing number of sequenced and annotated mitogenomes a coherent picture of why some genes were lost, or relocated to the nucleus, is emerging. In this review, we describe the characteristics of mitochondria-to-nucleus gene transfer and the resulting varied content of mitogenomes across eukaryotes. We introduce a 'burst-upon-drift' model to best explain nuclear-mitochondrial population genetics with flares of transfer due to genetic drift.
Keywords: CoRR hypothesis; Endosymbiont gene transfer; Evolutionary cell biology; Mitochondrial DNA; Mitochondrial evolution; Mitochondrial mutation rates.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
MitoCOGs: clusters of orthologous genes from mitochondria and implications for the evolution of eukaryotes.BMC Evol Biol. 2014 Nov 25;14:237. doi: 10.1186/s12862-014-0237-5. BMC Evol Biol. 2014. PMID: 25421434 Free PMC article.
-
Complete Mitochondrial Genomes of Ancyromonads Provide Clues for the Gene Content and Genome Structures of Ancestral Mitochondria.J Eukaryot Microbiol. 2025 May-Jun;72(3):e70012. doi: 10.1111/jeu.70012. J Eukaryot Microbiol. 2025. PMID: 40384044 Free PMC article.
-
Single-Cell Genomics Reveals the Divergent Mitochondrial Genomes of Retaria (Foraminifera and Radiolaria).mBio. 2023 Apr 25;14(2):e0030223. doi: 10.1128/mbio.00302-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939357 Free PMC article.
-
Evolution of mitochondrial gene content: gene loss and transfer to the nucleus.Mol Phylogenet Evol. 2003 Dec;29(3):380-95. doi: 10.1016/s1055-7903(03)00194-5. Mol Phylogenet Evol. 2003. PMID: 14615181 Review.
-
Clingy genes: Why were genes for ribosomal proteins retained in many mitochondrial genomes?Biochim Biophys Acta Bioenerg. 2020 Nov 1;1861(11):148275. doi: 10.1016/j.bbabio.2020.148275. Epub 2020 Jul 23. Biochim Biophys Acta Bioenerg. 2020. PMID: 32712152 Review.
Cited by
-
Assembly and comparative analysis of the complete mitochondrial of Spodiopogon sagittifolius, an endemic and protective species from Yunnan, China.BMC Plant Biol. 2025 Mar 24;25(1):373. doi: 10.1186/s12870-025-06341-z. BMC Plant Biol. 2025. PMID: 40122803 Free PMC article.
-
Assembly and analysis of the first complete mitochondrial genome sequencing of main Tea-oil Camellia cultivars Camellia drupifera (Theaceae): revealed a multi-branch mitochondrial conformation for Camellia.BMC Plant Biol. 2025 Jan 3;25(1):13. doi: 10.1186/s12870-024-05996-4. BMC Plant Biol. 2025. PMID: 39754047 Free PMC article.
-
Characterization of the Complete Mitochondrial Genome of the Red Alga Ahnfeltiopsis flabelliformis (Rhodophyta, Gigartinales, Phyllophoraceae) and Its Phylogenetic Analysis.Biology (Basel). 2025 May 30;14(6):638. doi: 10.3390/biology14060638. Biology (Basel). 2025. PMID: 40563889 Free PMC article.
-
The Nitroplast and Its Relatives Support a Universal Model of Features Predicting Gene Retention in Endosymbiont and Organelle Genomes.Genome Biol Evol. 2024 Jul 3;16(7):evae132. doi: 10.1093/gbe/evae132. Genome Biol Evol. 2024. PMID: 38900924 Free PMC article.
-
Diverse evolutionary trajectories of mitocoding DNA in mammalian and avian nuclear genomes.Genome Res. 2025 Jun 2;35(6):1313-1324. doi: 10.1101/gr.279428.124. Genome Res. 2025. PMID: 40164502
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

