Are the European reference networks for rare diseases ready to embrace machine learning? A mixed-methods study
- PMID: 38273306
- PMCID: PMC10809751
- DOI: 10.1186/s13023-024-03047-7
Are the European reference networks for rare diseases ready to embrace machine learning? A mixed-methods study
Abstract
Background: The delay in diagnosis for rare disease (RD) patients is often longer than for patients with common diseases. Machine learning (ML) technologies have the potential to speed up and increase the precision of diagnosis in this population group. We aim to explore the expectations and experiences of the members of the European Reference Networks (ERNs) for RDs with those technologies and their potential for application.
Methods: We used a mixed-methods approach with an online survey followed by a focus group discussion. Our study targeted primarily medical professionals but also other individuals affiliated with any of the 24 ERNs.
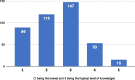
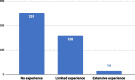
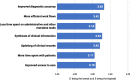
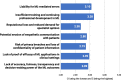
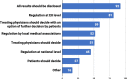
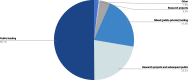
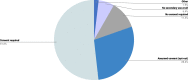
Results: The online survey yielded 423 responses from ERN members. Participants reported a limited degree of knowledge of and experience with ML technologies. They considered improved diagnostic accuracy the most important potential benefit, closely followed by the synthesis of clinical information, and indicated the lack of training in these new technologies, which hinders adoption and implementation in routine care. Most respondents supported the option that ML should be an optional but recommended part of the diagnostic process for RDs. Most ERN members saw the use of ML limited to specialised units only in the next 5 years, where those technologies should be funded by public sources. Focus group discussions concluded that the potential of ML technologies is substantial and confirmed that the technologies will have an important impact on healthcare and RDs in particular. As ML technologies are not the core competency of health care professionals, participants deemed a close collaboration with developers necessary to ensure that results are valid and reliable. However, based on our results, we call for more research to understand other stakeholders' opinions and expectations, including the views of patient organisations.
Conclusions: We found enthusiasm to implement and apply ML technologies, especially diagnostic tools in the field of RDs, despite the perceived lack of experience. Early dialogue and collaboration between health care professionals, developers, industry, policymakers, and patient associations seem to be crucial to building trust, improving performance, and ultimately increasing the willingness to accept diagnostics based on ML technologies.
Keywords: Artificial intelligence; Diagnosis; Diagnostic delay; European reference networks; Machine learning; Rare diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Decision No 1295/1999/EC of the European Parliament and of the Council of 29 April 1999 adopting a programme of Community action on rare diseases within the framework for action in the field of public health (1999 to 2003) (OJ L 155, 22.6.1999, p. 1). Decision repealed by Decision No 1786/2002/EC (OJ L 271, 9.10.2002, p. 1).
-
- Council Recommendation of 8 June 2009 on an action in the field of rare diseases (2009/C 151/02) (OJ C 151, 3.7.2009, pp. 7–10).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

