15 years of patient-reported outcomes in clinical trials leading to GU cancer drug approvals: a systematic review on the quality of data reporting and analysis
- PMID: 38273886
- PMCID: PMC10809115
- DOI: 10.1016/j.eclinm.2023.102413
15 years of patient-reported outcomes in clinical trials leading to GU cancer drug approvals: a systematic review on the quality of data reporting and analysis
Abstract
Background: Standardized, high-quality PRO data reporting is crucial for patient centered care in the field of oncology, especially in clinical trials that establish standard of care. This study evaluated PRO endpoint design, conduct and reporting methods in FDA approved drugs for GU malignancies.
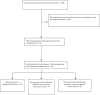
Methods: A systematic review of the FDA archives identified GU cancer drug approvals from Feb 2007 to July 2022. ClinicalTrials.gov and PubMed were used to retrieve relevant data. PRO data was screened, and analytic tools, interpretation methods in the published papers and study protocols were reviewed. Compliance with PRO reporting standards were assessed using PRO Endpoint Analysis Score (PROEAS), a 24-point scoring scale from Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium (SISAQOL).
Findings: We assessed 40 trial protocols with 27,011 participants, resulting in 14 renal cell cancer (RCC), 16 prostate cancer (PC), and 10 urothelial cancer (UC) approvals. PRO data was published for 27 trials, with 23 PRO publications (85%) focusing solely on PRO data, while 4 (15%) included PRO data in the original paper. Median time between primary clinical and secondary paper with PRO data was 10.5 months (range: 9-25 months). PROs were not planned as primary endpoints for any study but 14 (52%) reported them as secondary, 10 (37%) as exploratory outcomes, and 3 (11%) lacked any clarity on PRO data as endpoint. Mean PROEAS score of all GU cancers was 11.10 (range: 6-15), RCC (11.86, range: 6-15), UC (11.50, range: 9-14), and PC (10.56, range: 6-15). None met all the SISAQOL recommendations.
Interpretation: Low overall PROEAS score and delays in PRO data publication in GU cancer drug trials conducted in the past decade emphasize the need for improvement in quality of design and conduct of PRO endpoint in future trials and accelerated publication of PRO endpoints, using standardized analysis, and prespecified hypothesis driven endpoint. These improvements are essential for facilitating interpretation and application of PRO study findings to define patient care.
Funding: None.
Keywords: Patient reported outcomes; Prostate cancer; Renal cell cancer; Urothelial cancer.
© 2023 The Author(s).
Conflict of interest statement
Nothing to declare.
Figures

Similar articles
-
Patient-Reported Outcomes in Clinical Trials Leading to Cancer Immunotherapy Drug Approvals From 2011 to 2018: A Systematic Review.J Natl Cancer Inst. 2021 May 4;113(5):532-542. doi: 10.1093/jnci/djaa174. J Natl Cancer Inst. 2021. PMID: 33146385 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Use and Reporting of Patient-Reported Outcomes in Trials of Palliative Radiotherapy: A Systematic Review.JAMA Netw Open. 2022 Sep 1;5(9):e2231930. doi: 10.1001/jamanetworkopen.2022.31930. JAMA Netw Open. 2022. PMID: 36136335 Free PMC article.
-
Patient reported outcomes in the FDA approved drugs for systemic rheumatic diseases (2013-2024).Health Qual Life Outcomes. 2025 May 28;23(1):52. doi: 10.1186/s12955-025-02386-8. Health Qual Life Outcomes. 2025. PMID: 40437506 Free PMC article. Review.
-
Quality of patient-reported outcome reporting in randomised controlled trials of haematological malignancies according to international quality standards: a systematic review.Lancet Haematol. 2020 Dec;7(12):e892-e901. doi: 10.1016/S2352-3026(20)30292-1. Lancet Haematol. 2020. PMID: 33242446
Cited by
-
Health-related quality of life outcomes in randomized controlled trials in metastatic hormone-sensitive prostate cancer: a systematic review.EClinicalMedicine. 2024 Nov 13;78:102914. doi: 10.1016/j.eclinm.2024.102914. eCollection 2024 Dec. EClinicalMedicine. 2024. PMID: 39619239 Free PMC article. Review.
-
[Patient-reported outcomes-the role of the patient's subjective perspective for research and clinical care].Urologie. 2024 Sep;63(9):886-892. doi: 10.1007/s00120-024-02405-4. Epub 2024 Aug 7. Urologie. 2024. PMID: 39110186 Free PMC article. Review. German.
-
Understanding molecular mechanism of diabetic wound healing: addressing recent advancements in therapeutic managements.J Diabetes Metab Disord. 2025 Mar 6;24(1):76. doi: 10.1007/s40200-025-01588-7. eCollection 2025 Jun. J Diabetes Metab Disord. 2025. PMID: 40060271 Review.
-
Physical activity and quality of life in breast cancer survivors.Breast Dis. 2024;43(1):161-171. doi: 10.3233/BD-249005. Breast Dis. 2024. PMID: 38875025 Free PMC article.
-
The quality of life assessment of breast cancer patients.Breast Dis. 2024;43(1):173-185. doi: 10.3233/BD-249008. Breast Dis. 2024. PMID: 38875026 Free PMC article.
References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Powles T., Albiges L., Staehler M., et al. Updated European Association of Urology Guidelines: recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2018;73(3):311–315. - PubMed
-
- Flaig T.W., Spiess P.E., Agarwal N., et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18(3):329–354. - PubMed
-
- Escudier B., Motzer R.J., Sharma P., et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72(3):368–376. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

