A four-component reaction to access 3,3-disubstituted indolines via the palladium-norbornene-catalyzed ortho amination/ ipso conjunctive coupling
- PMID: 38274074
- PMCID: PMC10806727
- DOI: 10.1039/d3sc06409c
A four-component reaction to access 3,3-disubstituted indolines via the palladium-norbornene-catalyzed ortho amination/ ipso conjunctive coupling
Abstract
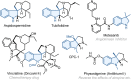
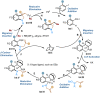
As an important class of multicomponent reactions, the palladium/norbornene (Pd/NBE) cooperative catalysis has been mainly restricted to the coupling of an aryl halide, an electrophile and a nucleophile. Here, we report the development of a Pd/NBE-catalyzed four-component reaction, which involves ortho C-H amination/ipso conjunctive coupling using an alkene and an external nucleophile. The use of alkene-tethered nitrogen electrophiles provides a rapid and modular synthesis of 3,3-disubstituted indolines from readily available aryl iodides. The reaction exhibits broad functional group tolerance, and its utility is exemplified in a streamlined formal synthesis of a rhodamine dye. Preliminary results of the asymmetric version of this reaction have also been obtained.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
-
For recent reviews on multi-component reactrions, see:
- Ugi I. Dömling A. Hörl W. Endeavour. 1994;18:115–122. doi: 10.1016/S0160-9327(05)80086-9. - DOI
- Ganem B. Acc. Chem. Res. 2009;42:463–472. doi: 10.1021/ar800214s. - DOI - PMC - PubMed
- Dömling A. Wang W. Wang K. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. - DOI - PMC - PubMed
-
-
-
For selected reviews on Pd/NBE cooperative catalysis, see:
- Catellani M. Synlett. 2003:0298–0313. doi: 10.1055/s-2003-37102. - DOI
- Catellani M., in Palladium in Organic Synthesis, ed. J. Tsuji, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, vol. 14, pp. 21–53
- Catellani M. Motti E. Della Ca' N. Acc. Chem. Res. 2008;41:1512–1522. doi: 10.1021/ar800040u. - DOI - PubMed
- Martins A., Mariampillai B. and Lautens M., in C–H Activation, ed. J.-Q. Yu and Z. Shi, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, vol. 292, pp. 1–33
- Ye J. Lautens M. Nat. Chem. 2015;7:863–870. doi: 10.1038/nchem.2372. - DOI - PubMed
- Della Ca' N. Fontana M. Motti E. Catellani M. Acc. Chem. Res. 2016;49:1389–1400. doi: 10.1021/acs.accounts.6b00165. - DOI - PubMed
- Liu Z. Gao Q. Cheng H. Zhou Q. Chem.–Eur. J. 2018;24:15461–15476. doi: 10.1002/chem.201802818. - DOI - PubMed
- Wang J. Dong G. Chem. Rev. 2019;119:7478–7528. doi: 10.1021/acs.chemrev.9b00079. - DOI - PMC - PubMed
- Zhao K. Ding L. Gu Z. Synlett. 2019;30:129–140. doi: 10.1055/s-0037-1611057. - DOI
- Li R. Dong G. J. Am. Chem. Soc. 2020;142:17859–17875. doi: 10.1021/jacs.0c09193. - DOI - PMC - PubMed
- Chen Z. Zhang F. Tetrahedron. 2023;134:133307. doi: 10.1016/j.tet.2023.133307. - DOI
-
-
- Wang J. Qin C. Lumb J.-P. Luan X. Chem. 2020;6:2097–2109.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

