Flexible Pectin Nanopatterning Drives Cell Wall Organization in Plants
- PMID: 38274264
- PMCID: PMC10806874
- DOI: 10.1021/jacsau.3c00616
Flexible Pectin Nanopatterning Drives Cell Wall Organization in Plants
Abstract
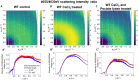
Plant cell walls are abundant sources of materials and energy. Nevertheless, cell wall nanostructure, specifically how pectins interact with cellulose and hemicelluloses to construct a robust and flexible biomaterial, is poorly understood. X-ray scattering measurements are minimally invasive and can reveal ultrastructural, compositional, and physical properties of materials. Resonant X-ray scattering takes advantage of compositional differences by tuning the energy of the incident X-ray to absorption edges of specific elements in a material. Using Tender Resonant X-ray Scattering (TReXS) at the calcium K-edge to study hypocotyls of the model plant, Arabidopsis thaliana, we detected distinctive Ca features that we hypothesize correspond to previously unreported Ca-Homogalacturonan (Ca-HG) nanostructures. When Ca-HG structures were perturbed by chemical and enzymatic treatments, cellulose microfibrils were also rearranged. Moreover, Ca-HG nanostructure was altered in mutants with abnormal cellulose, pectin, or hemicellulose content. Our results indicate direct structural interlinks between components of the plant cell wall at the nanoscale and reveal mechanisms that underpin both the structural integrity of these components and the molecular architecture of the plant cell wall.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Balat M.; Ayar G. Biomass energy in the world, use of biomass and potential trends. Energy sources 2005, 27 (10), 931–940. 10.1080/00908310490449045. - DOI
-
- Altartouri B.; Bidhendi A. J.; Tani T.; Suzuki J.; Conrad C.; Chebli Y.; Liu N.; Karunakaran C.; Scarcelli G.; Geitmann A. Pectin chemistry and cellulose crystallinity govern pavement cell morphogenesis in a multi-step mechanism. Plant Physiol. 2019, 181 (1), 127–141. 10.1104/pp.19.00303. - DOI - PMC - PubMed
-
- Dehors J.; Mareck A.; Kiefer-Meyer M. C.; Menu-Bouaouiche L.; Lehner A.; Mollet J. C. Evolution of Cell Wall Polymers in Tip-Growing Land Plant Gametophytes: Composition, Distribution, Functional Aspects and Their Remodeling. Front Plant Sci. 2019, 10, 441. 10.3389/fpls.2019.00441. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
