Altered O-linked glycosylation in benign and malignant meningiomas
- PMID: 38274327
- PMCID: PMC10809981
- DOI: 10.7717/peerj.16785
Altered O-linked glycosylation in benign and malignant meningiomas
Abstract
Background: Changes in protein glycosylation have been reported in various diseases, including cancer; however, the consequences of altered glycosylation in meningiomas remains undefined. We established two benign meningioma cell lines-SUT-MG12 and SUT-MG14, WHO grade I-and demonstrated the glycan and glycosyltransferase profiles of the mucin-type O-linked glycosylation in the primary benign meningioma cells compared with two malignant meningioma cell lines-HKBMM and IOMM-Lee, WHO grade III. Changes in O-linked glycosylation profiles in malignant meningiomas were proposed.
Methods: Primary culture technique, morphological analysis, and immunocytochemistry were used to establish and characterize two benign meningioma cell lines. The glycan profiles of the primary benign and malignant meningiomas cell lines were then analyzed using lectin cytochemistry. The gene expression of O-linked glycosyltransferases, mucins, sialyltransferases, and fucosyltransferases were analyzed in benign and malignant meningioma using the GEO database (GEO series GSE16581) and quantitative-PCR (qPCR).
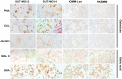
Results: Lectin cytochemistry revealed that the terminal galactose (Gal) and N-acetyl galactosamine (GalNAc) were highly expressed in primary benign meningioma cells (WHO grade I) compared to malignant meningioma cell lines (WHO grade III). The expression profile of mucin types O-glycosyltransferases in meningiomas were observed through the GEO database and gene expression experiment in meningioma cell lines. In the GEO database, C1GALT1-specific chaperone (COSMC) and mucin 1 (MUC1) were significantly increased in malignant meningiomas (Grade II and III) compared with benign meningiomas (Grade I). Meanwhile, in the cell lines, Core 2 β1,6-N-acetylglucosaminyltransferase-2 (C2GNT2) was highly expressed in malignant meningiomas. We then investigated the complex mucin-type O-glycans structures by determination of sialyltransferases and fucosyltransferases. We found ST3 β-galactoside α-2,3-sialyltransferase 4 (ST3GAL4) was significantly decreased in the GEO database, while ST3GAL1, ST3GAL3, α1,3 fucosyltransferases 1 and 8 (FUT1 and FUT8) were highly expressed in malignant meningioma cell lines-(HKBMM)-compared to primary benign meningioma cells-(SUT-MG12 and SUT-MG14).
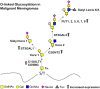
Conclusion: Our findings are the first to demonstrate the potential glycosylation changes in the O-linked glycans of malignant meningiomas compared with benign meningiomas, which may play an essential role in the progression, tumorigenesis, and malignancy of meningiomas.
Keywords: Fucosyltransferases; Glycosyltransferases; Meningiomas; Mucin; O-linked glycosylation; Sialyltransferases.
© 2024 Talabnin et al.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures



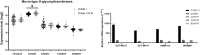

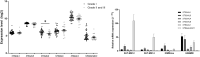

Similar articles
-
In vitro properties of four benign meningioma cells derived from WHO grade 1 meningiomas.Hum Cell. 2025 Apr 11;38(3):84. doi: 10.1007/s13577-025-01211-0. Hum Cell. 2025. PMID: 40214859
-
Pathways of mucin O-glycosylation in normal and malignant rat colonic epithelial cells reveal a mechanism for cancer-associated Sialyl-Tn antigen expression.Biol Chem. 2001 Feb;382(2):219-32. doi: 10.1515/BC.2001.029. Biol Chem. 2001. PMID: 11308020
-
Human B Cell Differentiation Is Characterized by Progressive Remodeling of O-Linked Glycans.Front Immunol. 2018 Dec 14;9:2857. doi: 10.3389/fimmu.2018.02857. eCollection 2018. Front Immunol. 2018. PMID: 30619255 Free PMC article.
-
Molecular Recognition of GalNAc in Mucin-Type O-Glycosylation.Acc Chem Res. 2023 Mar 7;56(5):548-560. doi: 10.1021/acs.accounts.2c00723. Epub 2023 Feb 23. Acc Chem Res. 2023. PMID: 36815693 Free PMC article. Review.
-
O-linked glycosylation in the mammary gland: changes that occur during malignancy.J Mammary Gland Biol Neoplasia. 2001 Jul;6(3):355-64. doi: 10.1023/a:1011331809881. J Mammary Gland Biol Neoplasia. 2001. PMID: 11547903 Review.
Cited by
-
Black rice bran‑derived anthocyanins attenuate cholangiocarcinoma cell migration via the alteration of epithelial‑mesenchymal transition and sialylation.Biomed Rep. 2024 Dec 6;22(2):28. doi: 10.3892/br.2024.1906. eCollection 2025 Feb. Biomed Rep. 2024. PMID: 39720294 Free PMC article.
-
In vitro properties of four benign meningioma cells derived from WHO grade 1 meningiomas.Hum Cell. 2025 Apr 11;38(3):84. doi: 10.1007/s13577-025-01211-0. Hum Cell. 2025. PMID: 40214859
-
Plasma extracellular vesicles proteomics in meningioma patients.Transl Oncol. 2024 Sep;47:102046. doi: 10.1016/j.tranon.2024.102046. Epub 2024 Jun 28. Transl Oncol. 2024. PMID: 38943923 Free PMC article.
References
-
- Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydrate Research. 2006;341(8):983–994. doi: 10.1016/j.carres.2006.02.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

