Treating Chronic, Intractable Pain with a Miniaturized Spinal Cord Stimulation System: 1-Year Outcomes from the AUS-nPower Study During the COVID-19 Pandemic
- PMID: 38274409
- PMCID: PMC10809818
- DOI: 10.2147/JPR.S436889
Treating Chronic, Intractable Pain with a Miniaturized Spinal Cord Stimulation System: 1-Year Outcomes from the AUS-nPower Study During the COVID-19 Pandemic
Abstract
Purpose: Spinal cord stimulation (SCS) is a highly effective treatment for chronic neuropathic pain. Despite recent advances in technology, treatment gaps remain. A small SCS system with a miniaturized implantable pulse generator (micro-IPG; <1.5 cm3 in volume) and an externally worn power source may be preferred by patients who do not want a large, implanted battery. We report here the long-term outcomes from the first-in-human study evaluating the safety and performance of a new neurostimulation system.
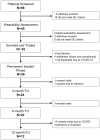
Patients and methods: This was a prospective, multi-center, open-label, single-arm study to evaluate this SCS system, in the treatment of chronic, intractable leg and low-back pain. Consented subjects who passed screening continued on to the long-term phase of the study. One-year, patient-reported outcomes (PRO's) such as pain (Numeric Rating Scale, NRS), functional disability, quality of life, and mood were captured.
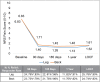
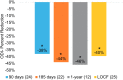
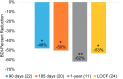
Results: Twenty-six (26) evaluable subjects with permanent implants were included in this analysis. The average leg pain NRS score decreased from 6.8 ± 1.2 at baseline to 1.1 ± 1.2 at the end of the study (p < 0.001), while the average low-back pain NRS score decreased from 6.8 ± 1.2 to 1.5 ± 1.2 (p < 0.001). The responder rate (proportion with ≥50% pain relief) was 91% in the leg(s) and 82% in the low back. There were significant improvements in functional disability (Oswestry Disability Index) and in mood (Beck Depression Inventory), demonstrating a 46% and 62% improvement, respectively (p < 0.001). Eleven-point Likert scales demonstrated the wearable to be very comfortable and very easy to use.
Conclusion: There were considerable challenges conducting a clinical study during the COVID-19 pandemic, such as missed study programming visits. Nevertheless, subjects had significant PRO improvements through 1-year. The small size of the implanted device, along with a proprietary waveform, may allow for improved SCS outcomes and a drop in incidence of IPG-pocket pain.
Keywords: FBSS; IPG; battery free; failed back surgery syndrome; implantable pulse generator; micro-IPG; neuropathic pain; persistent spinal pain syndrome; pulsed stimulation pattern.
© 2024 Salmon et al.
Conflict of interest statement
Drs. Salmon and Vahid Mohabbati have received financial assistance and fees for clinical trial support and costs associated with the implantation of Nalu medical SCS device. Dr. Salmon also has Nevro stock options and reports grants from PainCare Perth, during the conduct of the study. Dr. Makous is a consultant at Nalu Medical, BackStop Medical, Sella Therapies and ABVF, he owns stock in Nalu Medical and in addition has a patent US11090491B2 issued to Nalu Medical. Shilpa Kottalgi is an employee at Nalu Medical. Dr. Taverner is a consultant and lab instructor for Nevro, and he also is an unpaid board member of ANZCA and NSANZ. Dr. Bates was granted Nalu stock options, was compensated for flights and accommodations for speaking at NANS and is a consultant for Nalu and Abbott. Dr. Verrills was issued Nalu stock options. He sits on the DSMB and is a consultant for Saluda and Presidio. He also is paid honoraria for lectures on behalf of Saluda. Dr. Levy is an unpaid consultant for Biotronik, Nalu, Saluda and Abbott. He is INS past president and editor-in-chief of Neuromodulation. He has received stock option grants from Nalu and Saluda. Dr. Staats received royalties from Averitas and Qutenza Patch – he receives consulting fees from Saluda, Nalu, Vertos and Biotronik and honoraria from Medtronic. He is CMO for NSPC, sits on the DSMB of Nalu and electroCore and holds stock options in SPR, Nalu and Saluda. Dr. Heit is deceased. Drs. Mitchell, Du Toit, Yu and Green certified that they have no conflicting affiliations or involvements.
Figures

References
-
- Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Supplement):S13–S19. doi:10.1016/j.jpainsymman.2005.12.010 - DOI - PubMed
-
- Harmsen IE, Hasanova D, Elias GJB, et al. Trends in clinical trials for spinal cord stimulation. Stereotact Funct Neurosurg. 2020;27:1–12. - PubMed
-
- Karri J, Joshi M, Polson G, et al. Spinal cord stimulation for chronic pain syndromes: a review of considerations in practice management. Pain Physician. 2020;23(6):599–616. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

