COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign
- PMID: 38274635
- PMCID: PMC10810638
- DOI: 10.7759/cureus.52876
COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign
Retraction in
-
Retraction: COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign.Cureus. 2024 Feb 26;16(2):r137. doi: 10.7759/cureus.r137. eCollection 2024 Feb. Cureus. 2024. PMID: 38414517 Free PMC article.
Abstract
Our understanding of COVID-19 vaccinations and their impact on health and mortality has evolved substantially since the first vaccine rollouts. Published reports from the original randomized phase 3 trials concluded that the COVID-19 mRNA vaccines could greatly reduce COVID-19 symptoms. In the interim, problems with the methods, execution, and reporting of these pivotal trials have emerged. Re-analysis of the Pfizer trial data identified statistically significant increases in serious adverse events (SAEs) in the vaccine group. Numerous SAEs were identified following the Emergency Use Authorization (EUA), including death, cancer, cardiac events, and various autoimmune, hematological, reproductive, and neurological disorders. Furthermore, these products never underwent adequate safety and toxicological testing in accordance with previously established scientific standards. Among the other major topics addressed in this narrative review are the published analyses of serious harms to humans, quality control issues and process-related impurities, mechanisms underlying adverse events (AEs), the immunologic basis for vaccine inefficacy, and concerning mortality trends based on the registrational trial data. The risk-benefit imbalance substantiated by the evidence to date contraindicates further booster injections and suggests that, at a minimum, the mRNA injections should be removed from the childhood immunization program until proper safety and toxicological studies are conducted. Federal agency approval of the COVID-19 mRNA vaccines on a blanket-coverage population-wide basis had no support from an honest assessment of all relevant registrational data and commensurate consideration of risks versus benefits. Given the extensive, well-documented SAEs and unacceptably high harm-to-reward ratio, we urge governments to endorse a global moratorium on the modified mRNA products until all relevant questions pertaining to causality, residual DNA, and aberrant protein production are answered.
Keywords: autoimmune; cardiovascular; covid-19 mrna vaccines; gene therapy products; immunity; mortality; registrational trials; risk-benefit assessment; sars-cov-2 (severe acute respiratory syndrome coronavirus -2); serious adverse events.
Copyright © 2024, Mead et al.
Conflict of interest statement
Steve Kirsch is the founder of the Vaccine Safety Research Foundation or VSRF (vacsafety.org) but receives no income from this entity
Figures
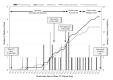
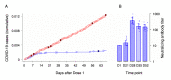

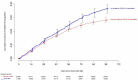


References
-
- Placebo use and unblinding in COVID-19 vaccine trials: recommendations of a WHO Expert Working Group. Singh JA, Kochhar S, Wolff J. Nat Med. 2021;27:569–570. - PubMed
-
- Mumps in the workplace. Further evidence of the changing epidemiology of a childhood vaccine-preventable disease. Kaplan KM, Marder DC, Cochi SL, et al. JAMA. 1988;260:1434–1438. - PubMed
-
- Vaccine Research & Development: How can COVID-19 vaccine development be done quickly and safely? [ Oct; 2023 ]. 2013. https://coronavirus.jhu.edu/vaccines/timeline https://coronavirus.jhu.edu/vaccines/timeline
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
