The potential role of Hippo pathway regulates cellular metabolism via signaling crosstalk in disease-induced macrophage polarization
- PMID: 38274792
- PMCID: PMC10808647
- DOI: 10.3389/fimmu.2023.1344697
The potential role of Hippo pathway regulates cellular metabolism via signaling crosstalk in disease-induced macrophage polarization
Abstract
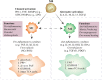
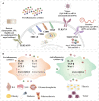
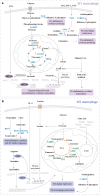
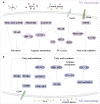
Macrophages polarized into distinct phenotypes play vital roles in inflammatory diseases by clearing pathogens, promoting tissue repair, and maintaining homeostasis. Metabolism serves as a fundamental driver in regulating macrophage polarization, and understanding the interplay between macrophage metabolism and polarization is crucial for unraveling the mechanisms underlying inflammatory diseases. The intricate network of cellular signaling pathway plays a pivotal role in modulating macrophage metabolism, and growing evidence indicates that the Hippo pathway emerges as a central player in network of cellular metabolism signaling. This review aims to explore the impact of macrophage metabolism on polarization and summarize the cell signaling pathways that regulate macrophage metabolism in diseases. Specifically, we highlight the pivotal role of the Hippo pathway as a key regulator of cellular metabolism and reveal its potential relationship with metabolism in macrophage polarization.
Keywords: Hippo; inflammatory diseases; macrophage polarization; metabolism; regulatory network.
Copyright © 2024 An, Tan, Yang, Gao and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

