Impaired macrophage and memory T-cell responses to Bacillus Calmette-Guerin nonpolar lipid extract
- PMID: 38274831
- PMCID: PMC10808680
- DOI: 10.3389/fimmu.2023.1263352
Impaired macrophage and memory T-cell responses to Bacillus Calmette-Guerin nonpolar lipid extract
Abstract
Introduction: The attenuation of BCG has led to the loss of not only immunogenic proteins but also lipid antigens.
Methods: Thus, we compared the macrophage and T-cell responses to nonpolar lipid extracts harvested from BCG and Mycobacterium tuberculosis (Mtb) to better understand the role of BCG lipids in the already known diminished responses of the vaccine strain.
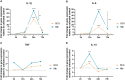
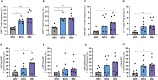
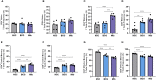
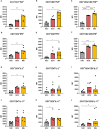
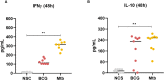
Results: Relative to Mtb, nonpolar lipid extract from BCG presented a reduced capacity to trigger the expression of the genes encoding TNF, IL-1b, IL-6 and IL-10 in RAW 264.7 macrophages. Immunophenotyping of PBMCs isolated from healthy individuals revealed that lipids from both BCG and Mtb were able to induce an increased frequency of CD4+ and CD8+ T cells, but only the lipid extract from Mtb enhanced the frequency of CD4-CD8-double-negative, γσ+, CD4+HLA-DR+, and γσ+HLA-DR+ T cells relative to the nonstimulated control. Interestingly, only the Mtb lipid extract was able to increase the frequency of CD4+ memory (CD45RO+) T cells, whereas the BCG lipid extract induced a diminished frequency of CD4+ central memory (CD45RO+CCR7-) T cells after 48 h of culture compared to Mtb.
Discussion: These findings show that the nonpolar lipids of the BCG bacilli presented diminished ability to trigger both proinflammatory and memory responses and suggest a potential use of Mtb lipids as adjuvants to increase the BCG vaccine efficacy.
Keywords: Bacillus Calmette-Guerin; Mycobacterium tuberculosis; macrophage gene expression; memory T-cell responses; nonpolar lipid extracts.
Copyright © 2024 Sarno, Leite, Augusto, Muller, de Ângelis, Pimentel, Queiroz and Arruda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO . Global tuberculosis report 2022. Geneva: World Health Organization. (2022).
-
- Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne J, et al. . Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette–Guérin vaccination against tuberculosis. Health Technol Assess (2013) 17:. doi: 10.3310/hta17370 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

