Intrinsic Burst-Blinking Nanographenes for Super-Resolution Bioimaging
- PMID: 38275287
- PMCID: PMC10910517
- DOI: 10.1021/jacs.3c11152
Intrinsic Burst-Blinking Nanographenes for Super-Resolution Bioimaging
Abstract
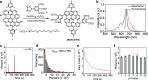
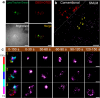
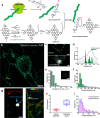
Single-molecule localization microscopy (SMLM) is a powerful technique to achieve super-resolution imaging beyond the diffraction limit. Although various types of blinking fluorophores are currently considered for SMLM, intrinsic blinking fluorophores remain rare at the single-molecule level. Here, we report the synthesis of nanographene-based intrinsic burst-blinking fluorophores for highly versatile SMLM. We image amyloid fibrils in air and in various pH solutions without any additive and lysosome dynamics in live mammalian cells under physiological conditions. In addition, the single-molecule labeling of nascent proteins in primary sensory neurons was achieved with azide-functionalized nanographenes via click chemistry. SMLM imaging reveals higher local translation at axonal branching with unprecedented detail, while the size of translation foci remained similar throughout the entire network. These various results demonstrate the potential of nanographene-based fluorophores to drastically expand the applicability of super-resolution imaging.
Conflict of interest statement
The authors declare the following competing financial interest(s): X. Liu, A. Narita, Q. Chen, S. Parekh, C. Cremer, K. Land-fester, K. Mllen, and M. Bonn are listed as inventors on a patent (PCT/EP2019/076496, WO2020070085) and Q. Chen, A. Narita, K. Mllen, S. Parken, M. Bonn and X. Liu are listed as inventors on a patent (PCT/EP2019/076497, WO 2020070086) related to the work presented in this manuscript. All other authors have nothing to disclose.
Figures

References
-
- Pujals S.; Feiner-Gracia N.; Delcanale P.; Voets I.; Albertazzi L. Super-Resolution Microscopy as a Powerful Tool to Study Complex Synthetic Materials. Nat. Rev. Chem. 2019, 3 (2), 68–84. 10.1038/s41570-018-0070-2. - DOI
-
- Wöll D.; Flors C. Super-Resolution Fluorescence Imaging for Materials Science. Small Methods 2017, 1 (10), 170019110.1002/smtd.201700191. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

