Diagnostic accuracy of commercially available serological tests for the detection of measles and rubella viruses: a systematic review and meta-analysis
- PMID: 38275299
- PMCID: PMC10865830
- DOI: 10.1128/jcm.01339-23
Diagnostic accuracy of commercially available serological tests for the detection of measles and rubella viruses: a systematic review and meta-analysis
Abstract
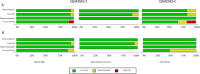
Measles and rubella serological diagnoses are done by IgM detection. The World Health Organization Global Measles and Rubella Laboratory Network previously endorsed Siemens Enzygnost enzyme-linked immunosorbant assay kits, which have been discontinued. A recommended replacement has not been determined. We aimed to search for suitable replacements by conducting a systematic review and meta-analysis of IgM detection methods that are currently available for measles and rubella. A systematic literature search was performed in Medline, Embase, Global Health, Cochrane Central, and Scopus on March 22 and on 27 September 2023. Studies reporting measles and/or rubella IgM detection with terms around diagnostic accuracy were included. Risk of bias was assessed using QUADAS tools. Meta-DiSc and R were used for statistical analysis. Clinical samples totalling 5,579 from 28 index tests were included in the measles meta-analysis. Sensitivity and specificity of the individual measles studies ranged from 0.50 to 1.00 and 0.53 to 1.00, respectively. Pooled sensitivity and specificity of all measles IgM detection methods were 0.94 (CI: 0.90-0.97) and 0.94 (CI: 0.91-0.97), respectively. Clinical samples totalling 4,983 from 15 index tests were included in the rubella meta-analysis. Sensitivity and specificity of the individual rubella studies ranged from 0.78 to 1.00 and 0.52 to 1.00, respectively. Pooled sensitivity and specificity of all rubella IgM detection methods were 0.97 (CI: 0.93-0.98) and 0.96 (CI: 0.93-0.98), respectively. Although more studies would be ideal, our results may provide valuable information when selecting IgM detection methods for measles and/or rubella.
Keywords: measles IgM; meta-analysis; rubella IgM; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- 2023. World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail
-
- Global measles and rubella strategic plan: 2012–2020. 2012. World Health Organization. Available from: https://apps.who.int/iris/rest/bitstreams/53400/retrieve
-
- Moss WJ, Shendale S, Lindstrand A, O’Brien AL, Turner N, Goodman T, Kretsinger K. 2021. SAGE working group on measles and rubella vaccines, measles and rubella eradication feasibility assessment workshop participants. 2021. feasibility assessment of measles and rubella eradication. Vaccine 39:3544–3559. - PubMed
-
- World Health Organization . 2013. Framework for verifying elimination of measles and rubella. Wkly Epidemiol Rec 88:89–99. https://www.who.int/publications/i/item/framework-for-verifying-eliminat.... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

