Performance Evaluation of the STANDARD i-Q COVID-19 Ag Test with Nasal and Oral Swab Specimens from Symptomatic Patients
- PMID: 38275478
- PMCID: PMC10814936
- DOI: 10.3390/diagnostics14020231
Performance Evaluation of the STANDARD i-Q COVID-19 Ag Test with Nasal and Oral Swab Specimens from Symptomatic Patients
Abstract
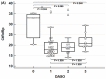
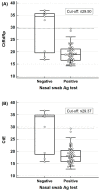
We evaluated the diagnostic performance of the STANDARD i-Q COVID-19 Ag Test, which was developed to detect viral antigens, using nasal and oral swabs. Sixty positive and 100 negative samples were analyzed. We determined the distribution of the Ct values according to the day of sample collection after symptom onset, the diagnostic performance of the total samples and subgroups separated by Ct value or time of sample collection, and the Ct value at which maximal accuracy was expected. No differences were observed in Ct values, except for the samples obtained on the day of symptom onset. The diagnostic sensitivity and specificity of the oral swabs were 75.0 and 100.0%, respectively, whereas those of the nasal swabs were 85.0 and 98.0%, respectively. The sensitivity was higher in samples with a high viral load collected earlier than those collected later, although the difference was not significant. False-negative results were confirmed in all samples with a Ct value ≥ 30.0. These results indicate that tests using oral and nasal swabs are helpful for diagnosing acute symptomatic cases with suspected high viral loads. Our tests exhibited relatively low sensitivity but high specificity rates, indicating the need to assess negative antigen test results.
Keywords: COVID-19; SARS-CoV-2 rapid antigen test; nasal swab; oral swab; performance evaluation; point-of-care testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation of the Diagnostic Accuracy of Nasal Cavity and Nasopharyngeal Swab Specimens for SARS-CoV-2 Detection via Rapid Antigen Test According to Specimen Collection Timing and Viral Load.Diagnostics (Basel). 2022 Mar 14;12(3):710. doi: 10.3390/diagnostics12030710. Diagnostics (Basel). 2022. PMID: 35328263 Free PMC article.
-
Viral load may impact the diagnostic performance of nasal swabs in nucleic acid amplification test and quantitative antigen test for SARS-CoV-2 detection.J Infect Chemother. 2022 Nov;28(11):1590-1593. doi: 10.1016/j.jiac.2022.07.023. Epub 2022 Aug 8. J Infect Chemother. 2022. PMID: 35953013 Free PMC article.
-
Comparison between Nasal and Nasopharyngeal Swabs for SARS-CoV-2 Rapid Antigen Detection in an Asymptomatic Population, and Direct Confirmation by RT-PCR from the Residual Buffer.Microbiol Spectr. 2022 Feb 23;10(1):e0245521. doi: 10.1128/spectrum.02455-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171010 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors.PLoS Med. 2022 May 26;19(5):e1004011. doi: 10.1371/journal.pmed.1004011. eCollection 2022 May. PLoS Med. 2022. PMID: 35617375 Free PMC article.
References
-
- World Health Organization . Weekly Epidemiological Update on COVID-19. 141st ed. World Health Organization; Geneva, Switzerland: 2023.
-
- National Institutes of Health Testing for SARS-CoV-2 Infection. [(accessed on 8 May 2023)]; Available online: https://www.covid19treatmentguidelines.nih.gov/overview/sars-cov-2-testing/
-
- Centers for Disease Control and Prevention Nucleic Acid Amplification Tests (NAATs) [(accessed on 3 May 2023)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html.
-
- Oh S.M., Lee J.S., Jo H.J., Kim D., Park D., Hwang Y.H., Choi Y., Lee C.M., Lee S., Chang E., et al. Clinical application of the Panbio COVID-19 Ag rapid test device and SSf-COVID19 kit for the detection of SARS-CoV-2 infection. BMC Res. Notes. 2022;15:357. doi: 10.1186/s13104-022-06226-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

