Novel Homozygous FA2H Variant Causing the Full Spectrum of Fatty Acid Hydroxylase-Associated Neurodegeneration (SPG35)
- PMID: 38275596
- PMCID: PMC10815826
- DOI: 10.3390/genes15010014
Novel Homozygous FA2H Variant Causing the Full Spectrum of Fatty Acid Hydroxylase-Associated Neurodegeneration (SPG35)
Abstract
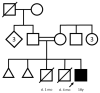
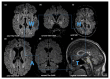
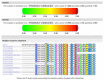
Fatty acid hydroxylase-associated neurodegeneration (FAHN/SPG35) is caused by pathogenic variants in FA2H and has been linked to a continuum of specific motor and non-motor neurological symptoms, leading to progressive disability. As an ultra-rare disease, its mutational spectrum has not been fully elucidated. Here, we present the prototypical workup of a novel FA2H variant, including clinical and in silico validation. An 18-year-old male patient presented with a history of childhood-onset progressive cognitive impairment, as well as progressive gait disturbance and lower extremity muscle cramps from the age of 15. Additional symptoms included exotropia, dystonia, and limb ataxia. Trio exome sequencing revealed a novel homozygous c.75C>G (p.Cys25Trp) missense variant in the FA2H gene, which was located in the cytochrome b5 heme-binding domain. Evolutionary conservation, prediction models, and structural protein modeling indicated a pathogenic loss of function. Brain imaging showed characteristic features, thus fulfilling the complete multisystem neurodegenerative phenotype of FAHN/SPG35. In summary, we here present a novel FA2H variant and provide prototypical clinical findings and structural analyses underpinning its pathogenicity.
Keywords: FA2H; FAHN; SPG35; genetic variant modeling; hereditary spastic paraplegia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

