Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis
- PMID: 38275741
- PMCID: PMC10294542
- DOI: 10.1002/14651858.CD007419.pub7
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis
Abstract
Background: Diabetic macular oedema (DMO) is a common complication of diabetic retinopathy. Antiangiogenic therapy with anti-vascular endothelial growth factor (anti-VEGF) can reduce oedema, improve vision, and prevent further visual loss. These drugs have replaced laser photocoagulation as the standard of care for people with DMO. In the previous update of this review, we found moderate-quality evidence that, at 12 months, aflibercept was slightly more effective than ranibizumab and bevacizumab for improving vision in people with DMO, although the difference may have been clinically insignificant (less than 0.1 logarithm of the minimum angle of resolution (logMAR), or five Early Treatment Diabetic Retinopathy Study (ETDRS) letters, or one ETDRS line).
Objectives: The objective of this updated review was to compare the effectiveness and safety of the different anti-VEGF drugs in RCTs at longer followup (24 months).
Search methods: We searched various electronic databases on 8 July 2022.
Selection criteria: We included randomised controlled trials (RCTs) that compared any anti-angiogenic drug with an anti-VEGF mechanism of action versus another anti-VEGF drug, another treatment, sham, or no treatment in people with DMO.
Data collection and analysis: We used standard Cochrane methods for pairwise meta-analysis and we augmented this evidence using network meta-analysis (NMA) methods. We used the Stata 'network' meta-analysis package for all analyses. We used the CINeMA (Confidence in Network Meta-Analysis) web application to grade the certainty of the evidence.
Main results: We included 23 studies (13 with industry funding) that enrolled 3513 people with DMO (median central retinal thickness (CRT) 460 microns, interquartile range (IQR) 424 to 482) and moderate vision loss (median best-corrected visual acuity (BCVA) 0.48 logMAR, IQR 0.42 to 0.55. One study that investigated ranibizumab versus sham and one study that mainly enrolled people with subclinical DMO and normal BCVA were not suitable for inclusion in the efficacy NMA. Consistent with the previous update of this review, we used ranibizumab as the reference drug for efficacy, and control (including laser, observation, and sham) as the reference for systemic safety. Eight trials provided data on the primary outcome (change in BCVA at 24 months, in logMAR: lower is better). We found no evidence of a difference between the following interventions and ranibizumab alone: aflibercept (mean difference (MD) -0.05 logMAR, 95% confidence interval (CI) -0.12 to 0.02; moderate certainty); bevacizumab (MD -0.01 logMAR, 95% CI -0.13 to 0.10; low certainty), brolucizumab (MD 0.00 logMAR, 95% CI -0.08 to 0.07; low certainty), ranibizumab plus deferred laser (MD 0.00 logMAR, 95% CI -0.11 to 0.10; low certainty), and ranibizumab plus prompt laser (MD 0.03 logMAR, 95% CI -0.04 to 0.09; very low certainty). We also analysed BCVA change at 12 months, finding moderate-certainty evidence of increased efficacy with brolucizumab (MD -0.07 logMAR, 95%CI -0.10 to -0.03 logMAR), faricimab (MD -0.08 logMAR, 95% CI -0.12 to -0.05), and aflibercept (MD -0.07 logMAR, 95 % CI -0.10 to -0.04) compared to ranibizumab alone, but the difference could be clinically insignificant. Compared to ranibizumab alone, NMA of six trials showed no evidence of a difference with aflibercept (moderate certainty), bevacizumab (low certainty), or ranibizumab with prompt (very low certainty) or deferred laser (low certainty) regarding improvement by three or more ETDRS lines at 24 months. There was moderate-certainty evidence of greater CRT reduction at 24 months with brolucizumab (MD -23 microns, 95% CI -65 to -1 9) and aflibercept (MD -26 microns, 95% CI -53 to 0.9) compared to ranibizumab. There was moderate-certainty evidence of lesser CRT reduction with bevacizumab (MD 28 microns, 95% CI 0 to 56), ranibizumab plus deferred laser (MD 63 microns, 95% CI 18 to 109), and ranibizumab plus prompt laser (MD 72 microns, 95% CI 25 to 119) compared with ranibizumab alone. Regarding all-cause mortality at the longest available follow-up (20 trials), we found no evidence of increased risk of death for any drug compared to control, although effects were in the direction of an increase, and clinically relevant increases could not be ruled out. The certainty of this evidence was low for bevacizumab (risk ratio (RR) 2.10, 95% CI 0.75 to 5.88), brolucizumab (RR 2.92, 95% CI 0.68 to 12.58), faricimab (RR 1.91, 95% CI 0.45 to 8.00), ranibizumab (RR 1.26, 95% CI 0.68 to 2.34), and very low for conbercept (RR 0.33, 95% CI 0.01 to 8.81) and aflibercept (RR 1.48, 95% CI 0.79 to 2.77). Estimates for Antiplatelet Trialists Collaboration arterial thromboembolic events at 24 months did not suggest an increase with any drug compared to control, but the NMA was overall incoherent and the evidence was of low or very low certainty. Ocular adverse events were rare and poorly reported and could not be assessed in NMAs.
Authors' conclusions: There is limited evidence of the comparative efficacy and safety of anti-VEGF drugs beyond one year of follow-up. We found no clinically important differences in visual outcomes at 24 months in people with DMO, although there were differences in CRT change. We found no evidence that any drug increases all-cause mortality compared to control, but estimates were very imprecise. Evidence from RCTs may not apply to real-world practice, where people in need of antiangiogenic treatment are often under-treated, and the individuals exposed to these drugs may be less healthy than trial participants.
Keywords: Angiogenesis Inhibitors [adverse effects, *therapeutic use]; Aptamers, Nucleotide [adverse effects, therapeutic use]; Bevacizumab [adverse effects, therapeutic use]; Diabetic Retinopathy [*complications]; Laser Coagulation [methods]; Macular Edema [*drug therapy, etiology, surgery]; Network Meta-Analysis; Quality of Life; Randomized Controlled Trials as Topic; Ranibizumab [adverse effects, therapeutic use]; Receptors, Vascular Endothelial Growth Factor [therapeutic use]; Recombinant Fusion Proteins [adverse effects, therapeutic use]; Triamcinolone [adverse effects, therapeutic use]; Vascular Endothelial Growth Factor A [*antagonists & inhibitors]; Visual Acuity [*drug effects, physiology].
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
GV: none known KC: none known EL: none known TP: received honoraria for advisory board and lecture fees from Bayer, Allergan, B-I, Sandoz, Roche, Novartis, Heidelberg, Zeiss, and Optos MP: received payment for participating on the Advisory Board for Allergan, Bayer and Novartis. Jennifer Evans (methods guide, not an author): none known Iris Gordon (search specialist, not an author): none known
Figures
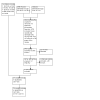
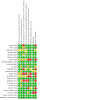
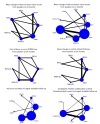









Update of
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2018 Oct 16;10(10):CD007419. doi: 10.1002/14651858.CD007419.pub6. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. PMID: 30325017 Free PMC article. Updated.
References
References to studies included in this review
Baker 2019 {published data only}
-
- Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA 2019;321(19):1880-94. - PMC - PubMed
BOLT 2010 {published data only}
-
- Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 2010;117(6):1078-86. - PubMed
-
- Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Archives of Ophthalmology 2012;130(8):972-9. - PubMed
Chatzirallis 2020 {published data only}
-
- Chatzirallis A, Theodossiadis P, Droutsas K, Koutsandrea C, Ladas I, Moschos MM. Ranibizumab versus aflibercept for diabetic macular edema: 18-month results of a comparative, prospective, randomized study and multivariate analysis of visual outcome predictors. Cutaneous and Ocular Toxicology 2020;39:317-22. - PubMed
DA VINCI 2011 {published data only}
-
- Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, et al. One-year outcomes of the DA VINCI Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology 2012;119(8):1658-65. - PubMed
-
- Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, et al. The DAVINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology 2011;118(9):1819-26. - PubMed
DRCRnet 2010 {published data only}
-
- Anonymous. Erratum: Patient-reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: Randomized clinical trial (JAMA Ophthalmology (2013) 131:10 (1339-1347) DOI: 10.1001/jamaophthalmol.2013.4592). JAMA Ophthalmology 2013;131(12):1652. - PubMed
DRCRnet 2015 {published data only}
Ekinci 2014 {published data only}
-
- Ekinci M, Ceylan E, Cakici O, Tanyildiz B, Olcaysu O, Cagatay HH. Treatment of macular edema in diabetic retinopathy: Comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Review of Ophthalmology 2014;9(2):139-43.
KITE and KESTREL 2022 {published data only}
-
- Brown DM, Emanuelli A, Bandello F, Barranco JJ, Figueira J, Souied E, et al. KESTREL and KITE: 52-week results from two Phase III pivotal trials of brolucizumab for diabetic macular edema. American Journal of Ophthalmology 2022;238:157-72. - PubMed
Li 2019 (REFINE) {published data only}
-
- Li X, Dai H, Li X, Han M, Li J, Suhner A, et al. Efficacy and safety of ranibizumab 0.5 mg in Chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefe's Archive for Clinical and Experimental Ophthalmology 2019;257(3):529-41. - PubMed
Liu 2022 {published data only}
LUCIDATE 2014 {published data only}
-
- Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). American Journal of Ophthalmology 2014;157(5):960-70. - PubMed
Nepomuceno 2013 {published data only}
-
- Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, et al. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. American Journal of Ophthalmology 2013;156(3):502-10. - PubMed
READ2 2009 {published data only}
-
- Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmology 2013;131(2):139-45. - PubMed
-
- Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology 2009;116(11):2175-81. - PubMed
-
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology 2010;117(11):2146-51. - PubMed
RELATION 2012 {unpublished data only}
-
- CRFB002DD13. A 12-month, two-armed, randomized, double-masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
-
- NCT01131585. A 12-month, two-armed, randomized, double-masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. clinicaltrials.gov/show/NCT01131585 (first received 25 May 2010).
RESOLVE 2010 {published data only}
-
- CRFB002D2201. A randomized, double-masked, multicenter, phase II study assessing the safety and efficacy of two concentrations of ranibizumab (intravitreal injections) compared with non-treatment control for the treatment of diabetic macular edema with center involvement. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
RESPOND 2013 {unpublished data only}
-
- Berger A, Sheidow T, Li R, Rehel B, De Takacsy F, Courseau AS. A Canadian 12-month, phase IIIb study of ranibizumab combination or monotherapy in visual impairment due to diabetic macular edema: Preliminary analysis ("RESPOND"). In: 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings; 2013 Oct 17-19; Montreal. 2013.
-
- CRFB002DCA05. A Canadian 12-month, prospective, randomized, open-label, multicenter, phase IIIb study assessing the efficacy, safety and cost of ranibizumab as combination and monotherapy in patients with visual impairment due to diabetic macular edema. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
RESTORE 2011 {published data only}
-
- Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 2013;120(10):2004-12. - PubMed
-
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615–62. - PubMed
-
- Mitchell P, Bressler N, Tolley K, Gallagher M, Petrillo J, Ferreira A, et al. Patient-reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmology 2013;131(10):1339-47. - PubMed
RETAIN 2016 {published data only}
REVEAL 2015 {published data only}
-
- Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 2015;122(7):1402-15. - PubMed
RISE and RIDE 2013 {published data only}
-
- Bressler NM, Varma R, Suñer IJ, Dolan CM, Ward J, Ehrlich JS, et al. Vision-related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISE. Ophthalmology 2014;121(12):2461-72. - PubMed
-
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013-22. - PubMed
-
- Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Archives of Ophthalmology 2012;130(9):1145-52. - PubMed
-
- Mieler WF, Kim JE, Yau L, Ehrlich JS. Earlier treatment is important in diabetic macular edema: Outcomes from phase III trials of intravitreal ranibizumab. In: 73rd Scientific sessions of the American Diabetes Association; 2013 Jun 21-25; Chicago. 2013.
-
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789-801. - PubMed
Soheilian 2007 {published data only}
-
- Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina 2012;32(2):314-21. - PubMed
-
- Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, et al. Intravitreal bevacizumab (Avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina 2007;27(9):1187-95. - PubMed
-
- Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116(6):1142-50. - PubMed
VIVID and VISTA 2015 {published data only}
-
- Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10):2044-52. - PubMed
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. ophthalmology 2014;121:2247-54. - PubMed
YOSEMITE and RHINE 2022 {published data only}
-
- Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE):two randomised, double-masked, phase 3 trials. Lancet 2022;399(10326):741-55. - PubMed
References to studies excluded from this review
Afridi 2016 (READ‐3) {published data only}
-
- Afridi R, Agarwal A, Sadiq MA, Aggarwal K, Hassan M, Soliman MK, et al. Effects of two different doses of Ranibizumab on the resolution and recurrence of diabetic macular edema in the Ranibizumab for edema of the macula in diabetes (READ-3) study. Investigative Ophthalmology and Visual Science 2016;57(12):ARVO E-abstract 2111.
Ahmadieh 2008 {published data only}
-
- Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(4):483-9. - PubMed
Ahmadieh 2013 {published data only}
-
- Ahmadieh H, Nourinia R, Hafezi-Moghadam A. Intravitreal fasudil combined with bevacizumab for persistent diabetic macular edema. JAMA Ophthalmology 2013;131(7):923-4. - PubMed
Azad 2012 {published data only}
BOULEVARD 2019 {published data only}
-
- Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology 2019;126(8):1155-70. - PubMed
BRDME 2020 {published data only}
-
- Vader MJ, Schauwvlieghe AM, Verbraak FD, Dijkman G, Hooymans JM, Los LI, et al. Bevacizumab and ranibizumab in diabetic macular edema study group. Comparing the efficacy of bevacizumab and ranibizumab in patients with diabetic macular edema (BRDME): the BRDME study, a randomized trial. Ophthalmology Retina 2020;4(8):777-8. - PubMed
Chen 2016 {published data only}
-
- Chen ZX, Fu JS, Song W, Wang CX, Zhang YL. Effect analysis of ranibizumab with laser photocoagulation therapy for diabetic macular edema. International Eye Science 2016;16(4):706-8.
ChiCTR‐TRC‐12002417 {unpublished data only}
-
- ChiCTR-TRC-12002417. A randomized controlled trial to compare the efficacy and safety of 1) macular laser vs. 2) repeated intravitreal bevacizumab vs. 3) combined repeated intravitreal bevacizumab with macular laser for diabetic macular edema. www2.ccrb.cuhk.edu.hk/registry/public/123 (accessed 17 September 2014).
Cornish 2018 (BEVORDEX) {published data only}
-
- Cornish EE, Teo KY, Gillies MC, Lim Ll, McAllister I, Sanmugasundram S, et al. Five year outcomes of the bevordex study (a multicenter randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 4847.
CRFB002DFR08 (LUDIC) {unpublished data only}
-
- CRFB002DFR08. Open-label, multicenter, study of the efficacy and safety of Lucentis® (ranibizumab 0.5 mg) in diabetic patients presenting a visual impairment due to diabetic macular edema in current medical practice (LUDIC). Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=9803 (accessed 28 May 2014).
CRFB002DGB14 (RELIGHT) {unpublished data only}
-
- CRFB002DGB14. RELIGHT - Ranibizumab treatment of diabetic macular oEdema with bimonthLy monItorinG after a pHase of initial Treatment. A UK, 18-month, prospective, open-label, multicentre, single-arm Phase IIIb study, with 12-month primary endpoint, assessing the efficacy and safety of Ranibizumab in patients with visual impairment. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/products.jsp?d... (accessed 28 May 2014).
CRFB002DNO02 (PTIMAL) {unpublished data only}
-
- CRFB002DNO02. An open-label, prospective, multicenter, uncontrolled, Proof of concept study assessing the efficacy of Lucentis (ranibizumab) administered by an individualized “treat and extend” dosing regimen in patients with visual impairment due to dIabetic macular edema. Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=10423 (accessed 28 May 2014).
Ding 2015 {published data only}
-
- Ding GP, Ding GL, Lei S, Shan WQ, Xie GJ, Ding XJ. Clinical effect of Conbercept intravitreal injection combined with 577nm micro-pulse laser on the treatment of diabetic macular edema. International Eye Science 2015;15(11):1942-4.
DRCRnet 2007 {published data only}
DRCRnet 2011 {published data only}
-
- Diabetic Retinopathy Clinical Research Network, Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011;31(6):1009-27. - PMC - PubMed
DRCRnet 2012 {published data only}
Eichenbaum 2018 {published data only}
-
- Eichenbaum DA, Duerr E, Patel HR, Pollack SM. Monthly versus treat-and-extend ranibizumab for diabetic macular edema: a prospective, randomized trial. Ophthalmic Surgery, Lasers and Imaging Retina 2018;49(11):e191-7. - PubMed
Faghihi 2008 {published data only}
-
- Faghihi H, Roohipoor R, Mohammadi SF, Hojat-Jalali K, Mirshahi A, Lashay A, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. European Journal of Ophthalmology 2008;18(6):941-8. - PubMed
Fang 2016 {published data only}
-
- Fang MX, Wu BB, Jia HJ. Efficacy of intravitreal injection of Bevacizumab with laser photocoagulation for diabetic macular edema. International Eye Science 2016;16(15):909-11.
Fouda 2017 {published data only}
Gillies 2014 (BEVORDEX) {published data only}
-
- Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 2014;121(12):2473-81. - PubMed
Gillies 2015 (BEVORDEX) {published data only}
-
- Gillies MC, Lim LL, Campain AE, Mehta H, Quin G, Fraser-Bell S, et al. BEVORDEX- A multicentre randomized clinical trial of intravitreal dexamethasone versus intravitreal bevacizumab for persistent diabetic macular edema. Investigative Ophthalmology and Visual Science 2015;56(7):ARVO E-abstract 3144.
Huang 2016 {published data only}
-
- Huang JD, Song ZY. Clinical study of grid pattern laser photocoagulation with ranibizumab for diabetic macular edema. International Eye Science 2016;16(3):493-5.
Ishibashi 2014 {published data only}
-
- Ishibashi T, Yuzawa M, Yoshimura N, Ohji M, Ishida S, Isogawa N, et al. Japan phase 3 study of pegaptanib sodium in patients with diabetic macular edema. Nippon Ganka Gakkai Zasshi 2014;118(9):773-82. - PubMed
-
- NCT01100307. A phase 3 study to compare the efficacy and safety of 0.3 mg pegaptanib sodium to sham Injections In subjects with diabetic macular edema [A phase 3, randomized, controlled, double-masked, multi-center, comparative, in parallel groups (for 24 weeks), to compare the efficacy and safety of 0.3 mg pegaptanib sodium, with sham injections, and open study (for 30 weeks) to confirm the safety of 0.3 mg pegaptanib sodium in subjects with diabetic macular edema (DME)]. clinicaltrials.gov/show/NCT01100307 (first received 7 April 2010).
Jovanovic 2015 {published data only}
-
- Jovanović S, Čanadanović V, Sabo A, Grgić Z, Mitrović M, Rakić D. Intravitreal bevacizumab injection alone or combined with macular photocoagulation compared to macular photocoagulation as primary treatment of diabetic macular edema. Vojnosanitetski Pregled 2015;72(10):876-82. - PubMed
Lafuente 2017 {published data only}
-
- Lafuente M, Ortín L, Argente M, Guindo JL, López-Bernal MD, López-Román, FJ, et al. Combined intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema: two-year randomized single-blind controlled trial results. Retina 2017;37(7):1277-86. - PubMed
-
- Lafuente M, Ortín L, Argente M, Guindo JL, López-Bernal MD, López-Román, FJ, et al. Three-year outcomes in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema. Retina 2019;39(6):1083-90. - PMC - PubMed
Li 2015 {published data only}
-
- Li S, Liu S, Liu L, Li J. Evaluation on efficacy and safety of combination treatment with intravitreal injection of ranibizumab and laser photocoagulation for diabetic macular edema. Journal of Jilin University Medicine Edition 2015;41(3):643-7.
Lopez‐Galvez 2014 {published and unpublished data}
-
- Lopez-Galvez MI, Arias L, Roura M. Efficacy and safety profile of ranibizumab versus laser photocoagulation in patients with diabetic macular edema. Re-Des Study. Ophthalmologica 2014;232:115.
Macugen 2005 {published data only}
-
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747-57. - PubMed
Macugen 2011 {published data only}
-
- Loftus JV, Sultan MB, Pleil AM, Macugen 1013 Study Group. Changes in vision and health-related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Investigative Ophthalmology and Visual Science 2011;52(10):7498-505. - PubMed
-
- Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS, Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011;118(6):1107-18. - PubMed
NCT00387582 {published data only}
-
- NCT00387582. Efficacy study of lucentis in the treatment of diabetic macular edema – a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema [Lucentis in the treatment of macular edema - a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema]. clinicaltrials.gov/show/NCT00387582 (first received 11 October 2006).
NCT00997191 (IBeTA) {published data only}
-
- NCT00997191. Intravitreal bevacizumab and intravitreal triamcinolone associated to laser photocoagulation for diabetic macular edema (IBeTA). clinicaltrials.gov/show/NCT00997191 (first received 15 October 2009).
NCT01445899 (MATISSE) {published data only}
-
- NCT01445899. PF-04523655 dose escalation study, and evaluation of PF-04523655 with/without ranibizumab in diabetic macular edema (DME) (MATISSE) [An open-label dose escalation study of PF-04523655 (Stratum I) combined with a prospective, randomized, double-masked, multi-center, controlled study (Stratum II) evaluating the efficacy and safety of PF-04523655 alone and in combination with ranibizumab versus ranibizumab alone in diabetic macular edema (MATISSE STUDY)]. clinicaltrials.gov/ct2/show/study/NCT01445899 (first received 30 September 2011).
NCT01565148 (IDEAL) {published data only}
-
- NCT01565148. A randomized, multi-center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo-007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema (the iDEAL Study) (iDEAL) [A randomized, multi-center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo-007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema with involvement of the foveAL center (the iDEAL Study)]. clinicaltrials.gov/ct2/show/study/NCT01565148 (first received 15 March 2012).
NCT01635790 (BRDME) {published data only}
-
- NCT01635790. Comparing the effectiveness and costs of bevacizumab to ranibizumab in patients with diabetic macular edema (BRDME) (BRDME). clinicaltrials.gov/ct2/show/NCT01635790 (first received 28 June 2012).
NCT02348918 {published data only}
-
- NCT02348918. A phase 2 randomized, controlled, double-masked, multicenter clinical trial designed to evaluate the safety and exploratory efficacy of Luminate® (ALG-1001) as compared to Avastin® and focal laser photocoagulation in the treatment of diabetic macular edema. clinicaltrials.gov/ct2/show/NCT02348918 (first received 12 January 2015).
NCT02645734 {published data only}
-
- NCT02645734. The effect of bevacizumab and ziv-aflibercept in diabetic macular edema [The comparison between the therapeutic effect of intravitreal bevacizumab and ziv-aflibercept in diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02645734 (first received 2 January 2016).
NCT02712008 {published data only}
-
- NCT02712008. Anti-vasculaR endothelial growth factor plUs anti-angiopoietin 2 in fixed comBination therapY: evaluation for the treatment of diabetic macular edema [A randomized, double-masked, active-controlled, phase 2 study of the efficacy, safety, and tolerability of repeated doses of intravitreal REGN910-3 in patients with diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02712008 (first received 14 March 2016).
NCT02985619 (BEVATAAC) {published data only}
-
- NCT02985619. Bevacizumabe or triamcinolone for persistent diabetic macular edema (BEVATAAC) [Randomized trial evaluating bevacizumabe or triamcinolone for persistent diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02985619 (first received 1 December 2016).
Paccola 2008 {published data only}
-
- Paccola L, Costa RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). British Journal of Ophthalmology 2008;92(1):76-80. - PubMed
Payne 2021 {published data only}
-
- Payne JF, Wykoff CC, Clark WL, Bruce BB, Boyer DS, Brown DM, et al. Long-term outcomes of treat-and-extend ranibizumab with and without navigated laser for diabetic macular oedema: TREX-DME 3-year results. British Journal of Ophthalmology 2021;105(2):253-7. - PubMed
Soheilian 2015 {published data only}
-
- Soheilian M, Karimi S, Ramezani A, Montahai T, Yaseri M, Soheilian R, et al. Intravitreal diclofenac versus intravitreal bevacizumab in naive diabetic macular edema: a randomized double-masked clinical trial. International Ophthalmology 2015;35(3):421-8. - PubMed
Solaiman 2010 {published data only}
-
- Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina 2010;30(10):1638-45. - PubMed
Turkoglu 2015 {published data only}
-
- Turkoglu EB, Celık E, Aksoy N, Bursalı O, Ucak T, Alagoz G. Changes in vision related quality of life in patients with diabetic macular edema: ranibizumab or laser treatment? Journal of Diabetes and its Complications 2015;29(4):540-3. - PubMed
Wiley 2016 {published data only}
-
- Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-52. - PubMed
Zehetner 2013 {published data only}
-
- Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. British Journal of Ophthalmology 2013;97(4):454-9. - PubMed
References to ongoing studies
NCT04108156 (PAGODA) {published data only}
-
- NCT04108156. This study will evaluate the efficacy, safety, and pharmacokinetics of the port delivery system with ranibizumab in participants with diabetic macular edema compared with intravitreal ranibizumab (Pagoda) [A phase III, multicenter, randomized, visual assessor-masked, active-comparator study of the efficacy, safety, and pharmacokinetics of the port delivery system with ranibizumab in patients with diabetic macular edema (Pagoda)]. clinicaltrials.gov/ct2/show/NCT04108156 (first received 30 September 2019).
NCT04611152 (GLEAM) and NCT04603937 (GLIMMER) {published data only}
-
- NCT04603937. A study to evaluate the efficacy, durability, and safety of ksi-301 compared to aflibercept in participants with diabetic macular edema (DME) (GLIMMER) [A prospective, randomized, double-masked, active comparator-controlled, multi-center, two-arm, phase 3 study to evaluate the efficacy and safety of intravitreal ksi-301 compared with intravitreal aflibercept in participants with visual impairment secondary to treatment-naïve diabetic macular edema (DME)]. clinicaltrials.gov/ct2/show/NCT04603937 (first received 27 October 2020).
-
- NCT04611152. A trial to evaluate the efficacy, durability, and safety of ksi-301 compared to aflibercept in participants with diabetic macular edema (DME) (GLEAM) [A prospective, randomized, double-masked, active comparator-controlled, multi-center, two-arm, phase 3 study to evaluate the efficacy and safety of intravitreal ksi-301 compared with intravitreal aflibercept in participants with visual impairment secondary to treatment-naïve diabetic macular edema (DME)]. https://clinicaltrials.gov/ct2/show/NCT04611152 (first received 2 November 2020).
NCT05885503 (RHONE‐X) {unpublished data only}
-
- NCT05885503. A study to evaluate the long-term safety and tolerability of faricimab in participants with diabetic macular edema (Rhone-X) [A multicenter, open-label extension study to evaluate the long-term safety and tolerability of faricimab in patients with diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT04432831 (first received 16 June 2020).
Additional references
Aiello 2005
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. New England Journal of Medicine 2005;353(8):839-41. - PubMed
Antcliff 1999
-
- Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Seminars in Ophthalmology 1999;14(4):223-32. - PubMed
ATC 1994
Banfi 2013
-
- Banfi R, Attanasio F, Palazzi N, Colombini S, Falai T, Cecchi M, et al. Bevacizumab versus ranibizumab: why are we not playing the joker? International Journal of Clinical Pharmacy 2013;35(4):507-9. - PubMed
Baumal 2020
-
- Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John V, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology 2020;127(10):1345-59. - PubMed
Baumal 2021
-
- Baumal CR, Bodaghi B, Singer M, Tanzer DJ, Seres A, Joshi MR, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmology. Retina 2021;5(6):519-27. - PubMed
Borm 2009
-
- Borm GF, Lemmers O, Fransen J, Donders R. The evidence provided by a single trial is less reliable than its statistical analysis suggests. Journal of Clinical Epidemiology 2009;62(7):711-5. - PubMed
Brignardello‐Petersen 2019
-
- Brignardello-Petersen R, Mustafa RA, Siemieniuk RA, Murad MH, Agoritsas T, Izcovich A, et al. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. Journal of Clinical Epidemiology 2019;108:77-85. - PubMed
Brown 2004
-
- Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Archives of Ophthalmology 2004;122(3):330-5. - PubMed
Browning 2008
Campochiaro 2010
-
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102-12. - PubMed
CATT 2011
Chaimani 2013
Chaimani 2022
-
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ciulla 2003
-
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003;26(9):2653-64. - PubMed
Ciulla 2004
-
- Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Current Opinions in Ophthalmology 2004;15(3):211-20. - PubMed
Cunningham 2005
-
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747-57. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29:932-944. - PubMed
EMA 2022a
-
- European Medicines Agency. Ozurdex Product Information. Available at www.ema.europa.eu/en/medicines/human/EPAR/ozurdex (accessed 9 March 2023).
EMA 2022b
-
- European Medicines Agency. Lucentis Product Information. Available at www.ema.europa.eu/en/medicines/human/EPAR/lucentis (accessed 9 March 2023).
EMA 2023a
-
- European Medicines Agency. Eylea Product Information. Available at www.ema.europa.eu/en/medicines/human/EPAR/eylea (accessed 9 March 2023).
EMA 2023b
-
- European Medicines Agency. Beovu European public assessment report. Available at www.ema.europa.eu/en/medicines/human/EPAR/beovu (accessed 9 March 2023).
ETDRS 1985
-
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796-806. - PubMed
FDA 2011
-
- US Food and Drug Administration. EYLEA (afilbercept) injection label. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf (accessed 9 March 2023).
FDA 2014
-
- US Food and Drug Administration. LUCENTIS (ranibizumab injection) label. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2014/125156s105lbl.pdf (accessed 9 March 2023).
FDA 2019
-
- US Food and Drug Administration. Drug Approval Package: BEOVU (brolucizumab-dbll). Available at www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125_Orig1_toc.cfm (accessed 9 March 2023).
FDA 2022
-
- US Food and Drug Administration. VABYSMO Highlights of prescribing information. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2022/761235s000lbl.pdf (accessed 9 March 2023).
Frank 2004
-
- Frank RN. Diabetic retinopathy. New England Journal of Medicine 2004;350(1):48-58. - PubMed
Glanville 2006
Glassman 2020
Grover 2008
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. - PubMed
Haller 2010
-
- Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Archives of Ophthalmology 2010;128(3):289-96. - PubMed
Heier 2016
-
- Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmology 2016;134(1):95-9. - PubMed
Higgins 2011
-
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JAC, (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Hussain 2015
-
- Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert Opinion on Biological Therapy 2016;16(3):365-74. - PubMed
Jampol 2014
Jiang 2015
Khanani 2022
Klein 1984
-
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91(12):1464-74. - PubMed
Korobelnik 2014
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121(11):2247-54. - PubMed
Kuppermann 2010
-
- Kuppermann BD, Chou C, Weinberg DV, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Archives of Ophthalmology 2010;128(5):642-3. - PubMed
Li 2020
-
- Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 20220, Issue 5. Art. No: CD012208. [DOI: 10.1002/14651858.CD012208.pub2] - DOI - PMC - PubMed
Lois 2022
-
- Lois N, Campbell C, Waugh N, Azuara-Blanco A, Maredza M, Mistry H, et al, DIAMONDS study group. DIAbetic Macular Oedema aNd Diode Subthreshold micropulse laser (DIAMONDS): A randomized double-masked non-inferiority clinical trial. Ophthalmology 2023;130(1):14-27. - PubMed
Mbuagbaw 2017
Moja 2014
-
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD011230. [DOI: 10.1002/14651858.CD011230.pub2] - DOI - PMC - PubMed
Muston 2018
-
- Muston D, Korobelnik JF, Reason T, Hawkins N, Chatzitheofilou I, Ryan F, et al. An efficacy comparison of anti-vascular growth factor agents and laser photocoagulation in diabetic macular edema: a network meta-analysis incorporating individual patient-level data. BMC Ophthalmology 2018;18:340. - PMC - PubMed
Nikolakopoulou 2020
Olson 2013
Ontario HTA 2009
OZDRY 2015
Pagliarini 2014
-
- Pagliarini S, Beatty S, Lipkova B, Perez-Salvador Garcia E, Reynders S, et al. A 2-year, phase IV, multicentre, observational study of ranibizumab 0.5 mg in patients with neovascular age-related macular degeneration in routine clinical practice: the EPICOHORT study. Journal of Ophthalmology 2014;2014:Article ID 857148. [DOI: 10.1155/2014/857148] - DOI - PMC - PubMed
Parravano 2021
-
- Parravano M, Costanzo E, Scondotto G, Trifirò G, Virgili G. Anti-VEGF and other novel therapies for neovascular age-related macular degeneration: an update. BioDrugs 2021;35(6):673-92. - PubMed
Patrao 2016
-
- Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. American Journal of Ophthalmology 2016;172:51-7. - PubMed
Peto 2022
PLACID 2013
-
- Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120(9):1843-51. - PubMed
Reibaldi 2022
-
- Reibaldi M, Fallico M, Avitabile T, Marolo P, Parisi G, Cennamo G, et al. Frequency of intravitreal anti-VEGF injections and risk of death: a systematic review with meta-analysis. Ophthalmology. Retina 2022;6:369-76. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. - PubMed
Sarhoia 2022
-
- Sarohia GS, Nanji K, Khan M, Khalid MF, Rosenberg D, Deonarain DM, et al. Treat-and-extend versus alternate dosing strategies with anti-vascular endothelial growth factor agents to treat center involving diabetic macular edema: a systematic review and meta-analysis of 2,346 eyes. Survey of Ophthalmology 2022 [April 25 Epub ahead of print];67(5):1346-1363. [DOI: 10.1016/j.survophthal.2022.04.003] - DOI - PubMed
Schunemann 2022
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions.In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Stata 2021 [Computer program]
-
- Stata. Version 17. College Station, TX, USA: StataCorp, 2021.
Stewart 2016
-
- Stewart MW, Narayanan R, Gupta V, Rosenfeld PJ, Martin DF, Chakravarthy U. Counterfeit Avastin in India: punish the criminals, not the patients. American Journal of Ophthalmology 2016;170:228-31. - PubMed
Sugimoto 2022
TA274 2013
-
- National Institute for Clinical Excellence (NICE) - Technology appraisal guidance. Ranibizumab for treating diabetic macular oedema. www.nice.org.uk/guidance/ta274 (accessed 23 January 2023).
TA346 2015
-
- National Institute for Clinical Excellence (NICE) - Technology appraisal guidance. Aflibercept for treating diabetic macular oedema. www.nice.org.uk/guidance/ta346 (accessed 23 January 2023).
TA799 2022
-
- National Institute for Clinical Excellence (NICE) - Technology appraisal guidance. Faricimab for treating diabetic macular oedema. www.nice.org.uk/guidance/ta799 (accessed 23 January 2023).
Tranos 2004
-
- Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Survey of Ophthalmology 2004;49(5):470-90. - PubMed
Wang 2022
White 2015
-
- White IR. Network meta-analysis. Stata Journal 2015;15:951-85.
Yau 2012
Yepes‐Nuñez 2019
-
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. - PubMed
Zhang 2021
Ziemssen 2017
References to other published versions of this review
Parravano 2008
Parravano 2009
Virgili 2012
Virgili 2014
Virgili 2018
-
- Virgili G, Parravano M, Evans JR, Gordan I, Lucenteforte E, Cochrane Eyes and Vision Group. Anti‐vascular endothelial growth factor for diabetic macular oedema: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 10 CD007419. Art. No: CD007419. [DOI: 10.1002/14651858.CD007419.pub6] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

