AIBP: A New Safeguard against Glaucomatous Neuroinflammation
- PMID: 38275823
- PMCID: PMC10814024
- DOI: 10.3390/cells13020198
AIBP: A New Safeguard against Glaucomatous Neuroinflammation
Abstract
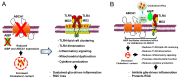
Glaucoma is a group of ocular diseases that cause irreversible blindness. It is characterized by multifactorial degeneration of the optic nerve axons and retinal ganglion cells (RGCs), resulting in the loss of vision. Major components of glaucoma pathogenesis include glia-driven neuroinflammation and impairment of mitochondrial dynamics and bioenergetics, leading to retinal neurodegeneration. In this review article, we summarize current evidence for the emerging role of apolipoprotein A-I binding protein (AIBP) as an important anti-inflammatory and neuroprotective factor in the retina. Due to its association with toll-like receptor 4 (TLR4), extracellular AIBP selectively removes excess cholesterol from the plasma membrane of inflammatory and activated cells. This results in the reduced expression of TLR4-associated, cholesterol-rich lipid rafts and the inhibition of downstream inflammatory signaling. Intracellular AIBP is localized to mitochondria and modulates mitophagy through the ubiquitination of mitofusins 1 and 2. Importantly, elevated intraocular pressure induces AIBP deficiency in mouse models and in human glaucomatous retina. AIBP deficiency leads to the activation of TLR4 in Müller glia, triggering mitochondrial dysfunction in both RGCs and Müller glia, and compromising visual function in a mouse model. Conversely, restoring AIBP expression in the retina reduces neuroinflammation, prevents RGCs death, and protects visual function. These results provide new insight into the mechanism of AIBP function in the retina and suggest a therapeutic potential for restoring retinal AIBP expression in the treatment of glaucoma.
Keywords: AAV; AIBP; TLR4; cholesterol; gene therapy; glaucoma; lipid rafts; mitochondria; neuroinflammation.
Conflict of interest statement
S.-H.C., W.-K.J. and Y.I.M. are co-inventors named on patents and patent applications by the University of California, San Diego. Y.I.M. is a scientific co-founder of Raft Pharmaceuticals LLC. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. Other authors declare no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

