B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19
- PMID: 38275936
- PMCID: PMC10820906
- DOI: 10.3390/v16010001
B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19
Abstract
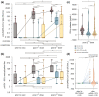
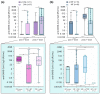
(1) Background: Vulnerable populations including transplant recipients are jeopardised by COVID-19. Herein, we report on B and T cell responses among liver and kidney organ recipients at our centre. (2) Methods: 23 liver and 45 kidney (14 thereof combined kidney/pancreas) transplanted patients were vaccinated with two doses of BNT162b2 followed by a booster dose of mRNA-1273 in 28 non-responders 4 months thereafter. Anti-SARS-CoV-2-Ig was measured by specific ELISA and virus neutralisation assay; T cell responses were measured by a spike protein-specific IFN-γ release assay. (3) Results: Compared to controls, B and T cell responses were weak in transplant recipients, particularly in those without prior exposure to SARS-CoV-2. Within this group, only 15% after the first and 58.3% after the second vaccination achieved seroconversion. A total of 14 out of 28 vaccination non-responders achieved a seroconversion after a third dose. Vaccination side effects were more frequent in healthy controls. The use of mycophenolate was associated with reduced anti-SARS-CoV-2-Ig production. (4) Conclusions: Our data confirm that vaccination responses are insufficient after standard vaccination in liver and kidney transplant recipients and are affected to a variable degree by specific immunosuppressants, particularly mycophenolate. Monitoring vaccination success and re-vaccinating those who are unresponsive seems prudent to achieve sufficient titres. Overall, prospective large-scale, multinational, multicentre studies or high-quality meta-analyses will be needed to generate personalised vaccination strategies in order to achieve protective immunity in high-risk, hard-to-immunize populations.
Keywords: COVID-19; SARS-CoV-2; T cell response; antibody response; kidney transplantation; liver transplantation; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients.J Hepatol. 2021 Aug;75(2):435-438. doi: 10.1016/j.jhep.2021.04.020. Epub 2021 Apr 21. J Hepatol. 2021. PMID: 33892006 Free PMC article.
-
Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients.J Hepatol. 2022 Jul;77(1):152-162. doi: 10.1016/j.jhep.2022.02.015. Epub 2022 Mar 10. J Hepatol. 2022. PMID: 35283215 Free PMC article.
-
Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients.Clin Res Cardiol. 2021 Aug;110(8):1142-1149. doi: 10.1007/s00392-021-01880-5. Epub 2021 Jul 9. Clin Res Cardiol. 2021. PMID: 34241676 Free PMC article.
-
Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients.J Am Soc Nephrol. 2021 Sep;32(9):2153-2158. doi: 10.1681/ASN.2021040490. Epub 2021 Jun 16. J Am Soc Nephrol. 2021. PMID: 34135083 Free PMC article.
-
Clinical application of COVID-19 vaccine in liver transplant recipients.Hepatobiliary Pancreat Dis Int. 2024 Aug;23(4):339-343. doi: 10.1016/j.hbpd.2023.08.010. Epub 2023 Aug 12. Hepatobiliary Pancreat Dis Int. 2024. PMID: 37620225 Review.
Cited by
-
Management of Kidney Transplant Outpatients With COVID-19: A Single Center Experience.Transpl Int. 2024 Sep 26;37:12920. doi: 10.3389/ti.2024.12920. eCollection 2024. Transpl Int. 2024. PMID: 39391264 Free PMC article.
References
-
- Amaeshi L.C. Navigating Through the Complications of Chronic Immunosuppression in Transplant Patients. Ann. Intern. Med. Clin. Cases. 2022;1:e220940C. doi: 10.7326/aimcc.2022.0940. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

