The Roles and Interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in Oral and Gastrointestinal Carcinogenesis: A Narrative Review
- PMID: 38276166
- PMCID: PMC10820765
- DOI: 10.3390/pathogens13010093
The Roles and Interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in Oral and Gastrointestinal Carcinogenesis: A Narrative Review
Abstract
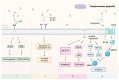
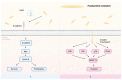
Epidemiological studies have spotlighted the intricate relationship between individual oral bacteria and tumor occurrence. Porphyromonas gingivalis and Fusobacteria nucleatum, which are known periodontal pathogens, have emerged as extensively studied participants with potential pathogenic abilities in carcinogenesis. However, the complex dynamics arising from interactions between these two pathogens were less addressed. This narrative review aims to summarize the current knowledge on the prevalence and mechanism implications of P. gingivalis and F. nucleatum in the carcinogenesis of oral squamous cell carcinoma (OSCC), colorectal cancer (CRC), and pancreatic ductal adenocarcinoma (PDAC). In particular, it explores the clinical and experimental evidence on the interplay between P. gingivalis and F. nucleatum in affecting oral and gastrointestinal carcinogenesis. P. gingivalis and F. nucleatum, which are recognized as keystone or bridging bacteria, were identified in multiple clinical studies simultaneously. The prevalence of both bacteria species correlated with cancer development progression, emphasizing the potential impact of the collaboration. Regrettably, there was insufficient experimental evidence to demonstrate the synergistic function. We further propose a hypothesis to elucidate the underlying mechanisms, offering a promising avenue for future research in this dynamic and evolving field.
Keywords: bateria–host interactions; colorectal cancers; microbial interaction; oral squamous cell carcinomas; pancreatic ductal carcinomas; periodontitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Escapa I.F., Chen T., Huang Y., Gajare P., Dewhirst F.E., Lemon K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems. 2018;3:e00187-18. doi: 10.1128/mSystems.00187-18. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

