A Three-Dimensional Model of Bacterial Biofilms and Its Use in Antimicrobial Susceptibility Testing
- PMID: 38276189
- PMCID: PMC10818914
- DOI: 10.3390/microorganisms12010203
A Three-Dimensional Model of Bacterial Biofilms and Its Use in Antimicrobial Susceptibility Testing
Abstract
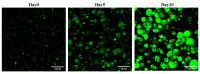
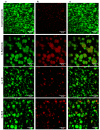
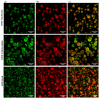
(1) Background: The discrepant antimicrobial susceptibility between planktonic and biofilm bacterial modes poses a problem for clinical microbiology laboratories and necessitates a relevant 3D experimental model allowing bacteria to grow in biofilm mode, in vitro, for use in anti-biofilm susceptibility testing. (2) Methods: This work develops a 3D biofilm model consisting of alginate beads containing S. aureus biofilm and encased within two thick layers of alginate matrix. The constructed model was placed on a thin Boyden chamber insert suspended on a 24-well culture plate containing the culture medium. The antibacterial activity of bacitracin and chlorhexidine digluconate (CD), either combined or separately, against 2D S. aureus culture was compared to that in the 3D biofilm model. Quantitative analysis and imaging analysis were performed by assessing the bacterial load within the matrix as well as measuring the optical density of the culture medium nourishing the matrix. (3) Results: The 3D biofilm model represented the typical complex characteristics of biofilm with greater insusceptibility to the tested antimicrobials than the 2D culture. Only bacitracin and CD in combination at 100× the concentration found to be successful against 2D culture were able to completely eliminate the 3D biofilm matrix. (4) Conclusions: The 3D biofilm model, designed to be more clinically relevant, exhibits higher antimicrobial insusceptibility than the 2D culture, demonstrating that the model might be useful for testing and discovering new antimicrobial therapies. The data also support the view that combination therapy might be the optimal approach to combat biofilm infections.
Keywords: 3D biofilm model; S. aureus biofilm; antimicrobial susceptibility testing; chronic wound infection; combination therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

