Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation
- PMID: 38276275
- PMCID: PMC10817655
- DOI: 10.3390/life14010146
Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation
Abstract
Background: Immune checkpoint inhibitors (ICI) are one of the most effective therapies in oncology, albeit associated with various immune-related adverse events also affecting the cardiovascular system.
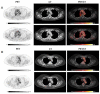
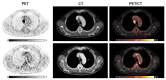
Methods: We aimed to investigate the effect of ICI on arterial 2-[18F]FDG uptake by using 2-[18F]FDG PET/CT imaging pre/post treatment in 47 patients with lung cancer. Maximum 2-[18F]FDG standardized uptake values (SUVmax) and target-to-background ratios (TBRs) were calculated along six arterial segments. We classified the arterial PET lesions by pre-existing active inflammation (cut-off: TBRpre ≥ 1.6). 2-[18F]FDG metabolic activity pre/post treatment was also quantified in bone marrow, spleen, and liver. Circulating blood biomarkers were additionally collected at baseline and after immunotherapy.
Results: ICI treatment resulted in significantly increased arterial inflammatory activity, detected by increased TBRs, in all arterial PET lesions analyzed. In particular, a significant elevation of arterial 2-[18F]FDG uptake was only recorded in PET lesions without pre-existing inflammation, in calcified as well as in non-calcified lesions. Furthermore, a significant increase in arterial 2-[18F]FDG metabolic activity after immunotherapy was solely observed in patients not previously treated with chemotherapy or radiotherapy as well as in those without CV risk factors. No significant changes were recorded in either 2-[18F]FDG uptake of bone marrow, spleen and liver after treatment, or the blood biomarkers.
Conclusions: ICI induces vascular inflammation in lung cancer patients lacking pre-existing arterial inflammation.
Keywords: 2-[18F]FDG; PET; atherosclerosis; cardio-oncology; immune checkpoint inhibitors; lung cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in the Large Arteries of Lymphoma Patients under 50 Years of Age.Biology (Basel). 2021 Nov 19;10(11):1206. doi: 10.3390/biology10111206. Biology (Basel). 2021. PMID: 34827199 Free PMC article.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Longitudinal Assessment of Subclinical Arterial Inflammation in Patients Receiving Immune Checkpoint Inhibitors by Sequential [18F]FDG PET Scans.Circ Cardiovasc Imaging. 2025 Feb;18(2):e016851. doi: 10.1161/CIRCIMAGING.124.016851. Epub 2025 Feb 4. Circ Cardiovasc Imaging. 2025. PMID: 39902567
-
The value of FDG PET/CT imaging in outcome prediction and response assessment of lymphoma patients treated with immunotherapy: a meta-analysis and systematic review.Eur J Nucl Med Mol Imaging. 2022 Nov;49(13):4661-4676. doi: 10.1007/s00259-022-05918-2. Epub 2022 Aug 6. Eur J Nucl Med Mol Imaging. 2022. PMID: 35932329 Free PMC article.
-
Prognostic value of 18F-FDG PET/CT in patients with advanced or metastatic non-small-cell lung cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis.Front Immunol. 2022 Nov 17;13:1014063. doi: 10.3389/fimmu.2022.1014063. eCollection 2022. Front Immunol. 2022. PMID: 36466905 Free PMC article.
Cited by
-
The impact of immune checkpoint inhibition on atherosclerosis in cancer patients.Front Immunol. 2025 Jul 31;16:1604989. doi: 10.3389/fimmu.2025.1604989. eCollection 2025. Front Immunol. 2025. PMID: 40821806 Free PMC article. Review.
References
-
- Miller M.J., Foy K.C., Kaumaya P.T. Cancer immunotherapy: Present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 2013;15:166–176. - PubMed
LinkOut - more resources
Full Text Sources

