Analysis of Crude, Diverse, and Multiple Advanced Glycation End-Product Patterns May Be Important and Beneficial
- PMID: 38276293
- PMCID: PMC10819149
- DOI: 10.3390/metabo14010003
Analysis of Crude, Diverse, and Multiple Advanced Glycation End-Product Patterns May Be Important and Beneficial
Abstract
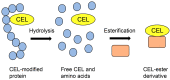
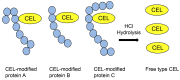
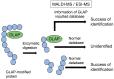
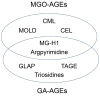
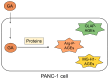
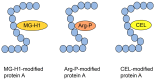
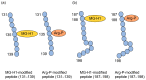
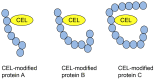
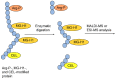
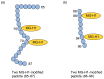
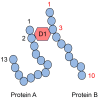
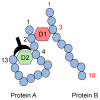
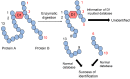
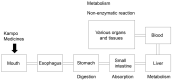
Lifestyle-related diseases (LSRDs), such as diabetes mellitus, cardiovascular disease, and nonalcoholic steatohepatitis, are a global crisis. Advanced glycation end-products (AGEs) have been extensively researched because they trigger or promote LSRDs. Recently, techniques such as fluorimetry, immunostaining, Western blotting, slot blotting, enzyme-linked immunosorbent assay, gas chromatography-mass spectrometry, matrix-assisted laser desorption-mass spectrometry (MALDI-MS), and electrospray ionization-mass spectrometry (ESI-MS) have helped prove the existence of intra/extracellular AGEs and revealed novel AGE structures and their modifications against peptide sequences. Therefore, we propose modifications to the existing categorization of AGEs, which was based on the original compounds identified by researchers in the 20th century. In this investigation, we introduce the (i) crude, (ii) diverse, and (iii) multiple AGE patterns. The crude AGE pattern is based on the fact that one type of saccharide or its metabolites or derivatives can generate various AGEs. Diverse and multiple AGE patterns were introduced based on the possibility of combining various AGE structures and proteins and were proven through mass analysis technologies such as MALDI-MS and ESI-MS. Kampo medicines are typically used to treat LSRDs. Because various compounds are contained in Kampo medicines and metabolized to exert effects on various organs or tissues, they may be suitable against various AGEs.
Keywords: Kampo medicines; advanced glycation end-products (AGEs); crude AGE pattern; diverse AGE pattern; electrospray ionization-mass spectrometry (ESI-MS); gas chromatography-mass spectrometry (GC-MS); lifestyle-related disease (LSRD); matrix-assisted laser desorption-mass spectrometry (MALDI-MS); multiple AGE pattern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Manfredelli D., Pariano M., Costantini C., Graziani A., Bozza S., Romani L., Puccetti P., Talesa V.N., Antgonelli C. Severe Acute Respiratory Syndrome Cornonavirus 2 (SARS-CoV-2) Spike Protein S1 Induces Methylglyoxa-Derived Hydroimidazolone/Receptor for Advanced Glycation End Products (MG-H1/RAGE) Activation to Promote Inflammation in Human Bronchial BEAS-2B Cells. Int. J. Mol. Sci. 2023;24:14868. doi: 10.3390/ijms241914868. - DOI - PMC - PubMed
-
- Bednarska K., Fecka I., Scheijen J.L.M., Ahles S., Vangrieken P., Schalkwijik C.G. A Citrus and Pomegranate Complex Reduces Methylglyoxal in Healthy Elderly Subjects: Secondary Analysis of a Double-Blind Randomized Cross-Over Clinical Trial. Int. J. Mol. Sci. 2023;24:13168. doi: 10.3390/ijms241713168. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

