Evaluation of the Immunosafety of Cucurbit[n]uril In Vivo
- PMID: 38276497
- PMCID: PMC10820314
- DOI: 10.3390/pharmaceutics16010127
Evaluation of the Immunosafety of Cucurbit[n]uril In Vivo
Abstract
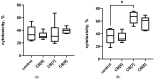
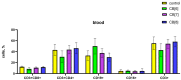
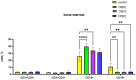
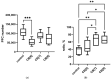
Cucurbiturils are a family of macrocyclic oligomers capable of forming host-guest complexes with various molecules. Due to noncovalent binding to drug molecules and low toxicity, cucurbiturils has been extensively investigated as potential carriers for drug delivery. However, the immune system's interactions with different drug carriers, including cucurbiturils, are still under investigation. In this study, we focused on cucurbiturils' immunosafety and immunomodulation properties in vivo. We measured blood counts and lymphocyte subpopulations in blood, spleen, and bone marrow, and assessed the in vivo toxicity to spleen and bone marrow cells after intraperitoneal administration to BALB/c mice. When assessing the effect of cucurbit[6]uril on blood parameters after three intraperitoneal injections within a week in laboratory animals, a decrease in white blood cells was found in mice after injections of cucurbit[6]util, but the observed decrease in the number of white blood cells was within the normal range. At the same time, cucurbit[7]uril and cucurbit[8]uril did not affect the leukocyte counts of mice after three injections. Changes in the number of platelets, erythrocytes, and monocytes, as well as in several other indicators, such as hematocrit or erythrocyte volumetric dispersion, were not detected. We show that cucurbiturils do not have immunotoxicity in vivo, with the exception of a cytotoxic effect on spleen cells after сucurbit[7]uril administration at a high dosage. We also evaluated the effect of cucurbiturils on cellular and humoral immune responses. We founded that cucurbiturils in high concentrations affect the immune system in vivo, and the action of various cucurbiturils differs in different homologues, which is apparently associated with different interactions in the internal environment of the body.
Keywords: blood cells; cucurbiturils; drug delivery; immune system; nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

