Drug Exposure and Susceptibility of Pyrazinamide Correlate with Treatment Response in Pyrazinamide-Susceptible Patients with Multidrug-Resistant Tuberculosis
- PMID: 38276514
- PMCID: PMC10820138
- DOI: 10.3390/pharmaceutics16010144
Drug Exposure and Susceptibility of Pyrazinamide Correlate with Treatment Response in Pyrazinamide-Susceptible Patients with Multidrug-Resistant Tuberculosis
Abstract
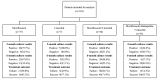
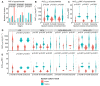
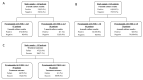
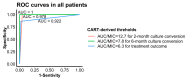
Exploring the influence of pyrazinamide exposure and susceptibility on treatment response is crucial for optimizing the management of multidrug-resistant tuberculosis (MDR-TB). This study aimed to investigate the association between pyrazinamide exposure, susceptibility, and response to MDR-TB treatment, as well as find clinical thresholds for pyrazinamide. A prospective multi-center cohort study of participants with MDR-TB using pyrazinamide was conducted in three TB-designated hospitals in China. Univariate and multivariate analyses were applied to investigate the associations. Classification and Regression Tree (CART) analysis was used to identify clinical thresholds, which were further evaluated by multivariate analysis and receiver operating characteristic (ROC) curves. The study included 143 patients with MDR-TB. The exposure/susceptibility ratio of pyrazinamide was associated with two-month culture conversion (adjusted risk ratio (aRR), 1.1; 95% confidence interval (CI), 1.07-1.20), six-month culture conversion (aRR, 1.1; 95% CI, 1.06-1.16), treatment success (aRR, 1.07; 95% CI, 1.03-1.10), as well as culture conversion time (adjusted hazard ratio (aHR) 1.18; 95% CI,1.14-1.23). The threshold for optimal improvement in sputum culture results at the sixth month of treatment was determined to be a pyrazinamide AUC0-24h/MIC ratio of 7.8. In conclusion, the exposure/susceptibility ratio of pyrazinamide is associated with the treatment response of MDR-TB, which may change in different Group A drug-based regimens.
Keywords: minimum inhibitory concentration; multidrug-resistant tuberculosis; pharmacokinetics; pyrazinamide; treatment outcome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Drug exposure and susceptibility of second-line drugs correlate with treatment response in patients with multidrug-resistant tuberculosis: a multicentre prospective cohort study in China.Eur Respir J. 2022 Mar 24;59(3):2101925. doi: 10.1183/13993003.01925-2021. Print 2022 Mar. Eur Respir J. 2022. PMID: 34737224 Free PMC article.
-
Pyrazinamide may improve fluoroquinolone-based treatment of multidrug-resistant tuberculosis.Antimicrob Agents Chemother. 2012 Nov;56(11):5465-75. doi: 10.1128/AAC.01300-12. Epub 2012 Aug 6. Antimicrob Agents Chemother. 2012. PMID: 22869570 Free PMC article.
-
Association between Regimen Composition and Treatment Response in Patients with Multidrug-Resistant Tuberculosis: A Prospective Cohort Study.PLoS Med. 2015 Dec 29;12(12):e1001932. doi: 10.1371/journal.pmed.1001932. eCollection 2015 Dec. PLoS Med. 2015. PMID: 26714320 Free PMC article.
-
Treatment of multidrug-resistant tuberculosis in Thailand.Chemotherapy. 1996;42 Suppl 3:10-5; discussion 30-3. doi: 10.1159/000239508. Chemotherapy. 1996. PMID: 8980862 Review.
-
'Z(S)-MDR-TB' versus 'Z(R)-MDR-TB': improving treatment of MDR-TB by identifying pyrazinamide susceptibility.Emerg Microbes Infect. 2012 Jul;1(7):e5. doi: 10.1038/emi.2012.18. Epub 2012 Jul 25. Emerg Microbes Infect. 2012. PMID: 26038418 Free PMC article. Review.
Cited by
-
Dynamic PET reveals compartmentalized brain and lung tissue antibiotic exposures of tuberculosis drugs.Nat Commun. 2024 Aug 14;15(1):6657. doi: 10.1038/s41467-024-50989-4. Nat Commun. 2024. PMID: 39143055 Free PMC article.
-
Dynamic PET Reveals Compartmentalized Brain and Lung Tissue Antibiotic Exposures.Res Sq [Preprint]. 2024 Mar 21:rs.3.rs-4096014. doi: 10.21203/rs.3.rs-4096014/v1. Res Sq. 2024. Update in: Nat Commun. 2024 Aug 14;15(1):6657. doi: 10.1038/s41467-024-50989-4. PMID: 38562706 Free PMC article. Updated. Preprint.
-
15-year trends in efficacy and effectiveness of treatment outcomes in drug-resistant pulmonary TB.IJTLD Open. 2025 Apr 9;2(4):187-198. doi: 10.5588/ijtldopen.24.0620. eCollection 2025 Apr. IJTLD Open. 2025. PMID: 40226134 Free PMC article.
References
-
- Aguilar Diaz J.M., Abulfathi A.A., Te Brake L.H., van Ingen J., Kuipers S., Magis-Escurra C., Raaijmakers J., Svensson E.M., Boeree M.J. New and Repurposed Drugs for the Treatment of Active Tuberculosis: An Update for Clinicians. Respiration. 2023;102:83–100. doi: 10.1159/000528274. - DOI - PMC - PubMed
-
- World Health Organization . WHO Treatment Guidelines for Drug-Resistant Tuberculosis. World Health Organization; Geneva, Switzerland: 2016. 2016 update.
-
- World Health Organization . WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Resistant Tuberculosis Treatment. World Health Organization; Geneva, Switzerland: 2020. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

