A Review of Rattlesnake Venoms
- PMID: 38276526
- PMCID: PMC10818703
- DOI: 10.3390/toxins16010002
A Review of Rattlesnake Venoms
Abstract
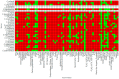
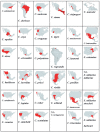
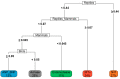
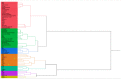
Venom components are invaluable in biomedical research owing to their specificity and potency. Many of these components exist in two genera of rattlesnakes, Crotalus and Sistrurus, with high toxicity and proteolytic activity variation. This review focuses on venom components within rattlesnakes, and offers a comparison and itemized list of factors dictating venom composition, as well as presenting their known characteristics, activities, and significant applications in biosciences. There are 64 families and subfamilies of proteins present in Crotalus and Sistrurus venom. Snake venom serine proteases (SVSP), snake venom metalloproteases (SVMP), and phospholipases A2 (PLA2) are the standard components in Crotalus and Sistrurus venom. Through this review, we highlight gaps in the knowledge of rattlesnake venom; there needs to be more information on the venom composition of three Crotalus species and one Sistrurus subspecies. We discuss the activity and importance of both major and minor components in biomedical research and drug development.
Keywords: COVID-19; Crotalus; Sistrurus; biomedical application; toxin; venom composition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fry B.G., Wickramaratna J.C., Hodgson W.C., Alewood P.F., Kini R., Ho H., Wüster W. Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: Taxonomic and toxinological implications. Rapid Commun. Mass. Spectrom. 2002;16:600–608. doi: 10.1002/rcm.613. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

