Thermodynamics of the Glassy Polymer State: Equilibrium and Non-Equilibrium Aspects
- PMID: 38276706
- PMCID: PMC10820664
- DOI: 10.3390/polym16020298
Thermodynamics of the Glassy Polymer State: Equilibrium and Non-Equilibrium Aspects
Abstract
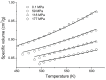
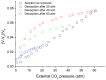
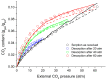
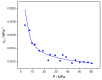
This work examines, first, the non-equilibrium character of the glassy state of polymer systems and its significance in the development of novel materials for important technological applications. Subsequently, it summarizes the essentials of the generalized lattice fluid approach for the description of this highly complex non-equilibrium behavior with an approximate and simple, yet analytically powerful formalism. The working equations are derived in a straightforward and consistent manner by clearly defining the universal and specific variables needed to describe the discussed properties. The role of the non-random distribution of molecular species and free volume in the glassy system is also examined, as is the role of strong specific interactions, such as hydrogen-bonding networks. This work also reports examples of applications in a variety of representative systems, including glass densification, retrograde vitrification, increase in glass-transition temperature in hydrogen-bonded polymer mixtures, and hysteresis phenomena in sorption-desorption from glassy polymer matrices.
Keywords: glass densification; glass transition; lattice-fluid; penetrant sorption; polymer swelling; statistical thermodynamics.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Gee G. The glassy state in polymers. Contemp. Phys. 1970;11:313–334. doi: 10.1080/00107517008204410. - DOI
-
- Hutchinson J.M. Relaxation processes and physical aging. In: Haward R.N., Young R.J., editors. The Physics of Glassy Polymers. 2nd ed. Springer; Berlin/Heidelberg, Germany: 1997.
-
- Minelli M., Sarti G.C. 110th Anniversary: Gas and Vapor Sorption in Glassy Polymeric Membranes—Critical Review of Different Physical and Mathematical Models. Ind. Eng. Chem. Res. 2020;59:341–365. doi: 10.1021/acs.iecr.9b05453. - DOI
-
- Roth C. Polymer glasses. In: Matyjaszewski K., Gnanou Y., Hadjichristidis N., Muthukumar M., editors. Macromolecular Engineering: From Precise Synthesis to Macroscopic Materials and Applications. 2nd ed. Wiley-VCH GmbH; Berlin, Germany: 2022.
-
- de Gennes P.G. Scaling Concepts in Polymer Physics. Cornell University Press; Ithaca, NY, USA: 1979.
Publication types
LinkOut - more resources
Full Text Sources

