Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism
- PMID: 38277137
- PMCID: PMC10824146
- DOI: 10.1080/19490976.2024.2304159
Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism
Abstract
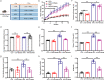
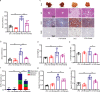
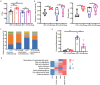
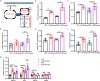
Gut microbiota plays an essential role in the progression of nonalcoholic fatty liver disease (NAFLD), making the gut-liver axis a potential therapeutic strategy. Bacteroides genus, the enriched gut symbionts, has shown promise in treating fatty liver. However, further investigation is needed to identify specific beneficial Bacteroides strains for metabolic disorders in NAFLD and elucidate their underlying mechanisms. In this study, we observed a positive correlation between the abundance of Bacteroides thetaiotaomicron (B. theta) and the alleviation of metabolic syndrome in the early and end stages of NAFLD. Administration of B. theta to HFD-fed mice for 12 weeks reduced body weight and fat accumulation, decreased hyperlipidemia and insulin resistance, and prevented hepatic steatohepatitis and liver injury. Notably, B. theta did not affect these indicators in low-fat diet (LFD)-fed mice and exhibited good safety. Mechanistically, B. theta regulated gut microbial composition, characterized by a decreased Firmicutes/Bacteroidetes ratio in HFD-Fed mice. It also increased gut-liver folate levels and hepatic metabolites, alleviating metabolic dysfunction. Additionally, treatment with B. theta increased the proportion of polyunsaturated fatty acid in the mouse liver, offering a widely reported benefit for NAFLD improvement. In conclusion, this study provides evidence that B. theta ameliorates NAFLD by regulating gut microbial composition, enhancing gut-liver folate and unsaturated fatty acid metabolism, highlighting the therapeutic role of B. theta as a potential probiotic for NAFLD.
Keywords: Bacteroides thetaiotaomicron; folate metabolism; gut microbiota; nonalcoholic fatty liver disease; unsaturated fatty acids.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, Kleiner DE, Brunt E, Bugianesi E, Yki-Järvinen H. International consensus panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi:10.1053/j.gastro.2019.11.312. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
