An improved conflict avoidance assay reveals modality-specific differences in pain hypersensitivity across sexes
- PMID: 38277178
- PMCID: PMC11090034
- DOI: 10.1097/j.pain.0000000000003132
An improved conflict avoidance assay reveals modality-specific differences in pain hypersensitivity across sexes
Abstract
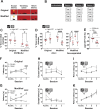
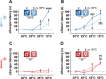
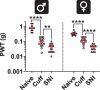
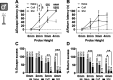
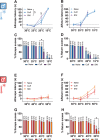
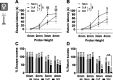
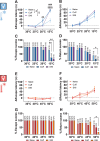
Abnormal encoding of somatosensory modalities (ie, mechanical, cold, and heat) are a critical part of pathological pain states. Detailed phenotyping of patients' responses to these modalities have raised hopes that analgesic treatments could one day be tailored to a patient's phenotype. Such precise treatment would require a profound understanding of the underlying mechanisms of specific pain phenotypes at molecular, cellular, and circuitry levels. Although preclinical pain models have helped in that regard, the lack of a unified assay quantifying detailed mechanical, cold, and heat pain responses on the same scale precludes comparing how analgesic compounds act on different sensory phenotypes. The conflict avoidance assay is promising in that regard, but testing conditions require validation for its use with multiple modalities. In this study, we improve upon the conflict avoidance assay to provide a validated and detailed assessment of all 3 modalities within the same animal, in mice. We first optimized testing conditions to minimize the necessary amount of training and to reduce sex differences in performances. We then tested what range of stimuli produce dynamic stimulus-response relationships for different outcome measures in naive mice. We finally used this assay to show that nerve injury produces modality-specific sex differences in pain behavior. Our improved assay opens new avenues to study the basis of modality-specific abnormalities in pain behavior.
Keywords: Cold; Conflict avoidance; Cuff; Female; Heat; Mechanical; Modality; Mouse; Neuropathic pain; Operant pain behavior; Preclinical; Preclinical pain models; Sex difference; Thermal; Translation; c57bl/6.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat.J Pain. 2008 Aug;9(8):739-49. doi: 10.1016/j.jpain.2008.03.008. Epub 2008 May 16. J Pain. 2008. PMID: 18486556 Free PMC article.
-
Measuring Pain Avoidance-Like Behavior in Drug-Dependent Rats.Curr Protoc Neurosci. 2018 Oct;85(1):e53. doi: 10.1002/cpns.53. Epub 2018 Sep 24. Curr Protoc Neurosci. 2018. PMID: 30248240 Free PMC article.
-
Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes.Pain. 2010 Sep;150(3):439-450. doi: 10.1016/j.pain.2010.05.002. Pain. 2010. PMID: 20627413
-
Between Scylla and Charybdis: assessing the multidimensional aspects of pain behaviors in rats using a double avoidance place preference paradigm.Pain. 2025 Mar 1;166(3):587-595. doi: 10.1097/j.pain.0000000000003383. Epub 2024 Aug 20. Pain. 2025. PMID: 39167457
-
Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain.Clin J Pain. 2009 Sep;25(7):632-40. doi: 10.1097/AJP.0b013e3181a68c64. Clin J Pain. 2009. PMID: 19692806 Review.
Cited by
-
Anxiety-Like Behaviors in Mice Unmasked: Revealing Sex Differences in Anxiety Using a Novel Light-Heat Conflict Test.J Neurosci Res. 2024 Dec;102(12):e70002. doi: 10.1002/jnr.70002. J Neurosci Res. 2024. PMID: 39654136
-
Painful Diabetic Neuropathy: Sex-Specific Mechanisms and Differences from Animal Models to Clinical Outcomes.Cells. 2024 Dec 7;13(23):2024. doi: 10.3390/cells13232024. Cells. 2024. PMID: 39682771 Free PMC article. Review.
-
Modulating Neural Circuits of Pain in Preclinical Models: Recent Insights for Future Therapeutics.Cells. 2024 Jun 7;13(12):997. doi: 10.3390/cells13120997. Cells. 2024. PMID: 38920628 Free PMC article. Review.
References
-
- Andersen HH, Poulsen JN, Uchida Y, Nikbakht A, Arendt-Nielsen L, Gazerani P. Cold and L-menthol-induced sensitization in healthy volunteers—a cold hypersensitivity analogue to the heat/capsaicin model. PAIN 2015;156:880–9. - PubMed
-
- Baron R, Dickenson AH, Calvo M, Dib-Hajj SD, Bennett DL. Maximizing treatment efficacy through patient stratification in neuropathic pain trials. Nat Rev Neurol 2023;19:53–64. - PubMed
-
- Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol 2012;11:999–1005. - PubMed
-
- Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede R-D; German Neuropathic Pain Research Network DFNS, and the EUROPAIN, and NEUROPAIN consortia. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. - PMC - PubMed
-
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 2008;12:591–9. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

