Transcranial Magnetic Stimulation of the Default Mode Network to Improve Sleep in Individuals With Insomnia Symptoms: Protocol for a Double-Blind Randomized Controlled Trial
- PMID: 38277210
- PMCID: PMC10858423
- DOI: 10.2196/51212
Transcranial Magnetic Stimulation of the Default Mode Network to Improve Sleep in Individuals With Insomnia Symptoms: Protocol for a Double-Blind Randomized Controlled Trial
Abstract
Background: Cortical hyperarousal and ruminative thinking are common aspects of insomnia that have been linked with greater connectivity in the default mode network (DMN). Therefore, disrupting network activity within the DMN may reduce cortical and cognitive hyperarousal and facilitate better sleep.
Objective: This trial aims to establish a novel, noninvasive method for treating insomnia through disruption of the DMN with repetitive transcranial magnetic stimulation, specifically with continuous theta burst stimulation (cTBS). This double-blind, pilot randomized controlled trial will assess the efficacy of repetitive transcranial magnetic stimulation as a novel, nonpharmacological approach to improve sleep through disruption of the DMN prior to sleep onset for individuals with insomnia. Primary outcome measures will include assessing changes in DMN functional connectivity before and after stimulation.
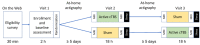
Methods: A total of 20 participants between the ages of 18 to 50 years with reported sleep disturbances will be recruited as a part of the study. Participants will then conduct an in-person screening and follow-on enrollment visit. Eligible participants then conduct at-home actigraphic collection until their first in-residence overnight study visit. In a double-blind, counterbalanced, crossover study design, participants will receive a 40-second stimulation to the left inferior parietal lobule of the DMN during 2 separate overnight in-residence visits. Participants are randomized to the order in which they receive the active stimulation and sham stimulation. Study participants will undergo a prestimulation functional magnetic resonance imaging scan and a poststimulation functional magnetic resonance imaging scan prior to sleep for each overnight study visit. Sleep outcomes will be measured using clinical polysomnography. After their first in-residence study visit, participants conduct another at-home actigraphic collection before returning for their second in-residence overnight study visit.
Results: Our study was funded in September 2020 by the Department of Defense (W81XWH2010173). We completed the enrollment of our target study population in the October 2022 and are currently working on neuroimaging processing and analysis. We aim to publish the results of our study by 2024. Primary neuroimaging outcome measures will be tested using independent components analysis, seed-to-voxel analyses, and region of interest to region of interest analyses. A repeated measures analysis of covariance (ANCOVA) will be used to assess the effects of active and sham stimulation on sleep variables. Additionally, we will correlate changes in functional connectivity to polysomnography-graded sleep.
Conclusions: The presently proposed cTBS protocol is aimed at establishing the initial research outcomes of the effects of a single burst of cTBS on disrupting the network connectivity of the DMN to improve sleep. If effective, future work could determine the most effective stimulation sites and administration schedules to optimize this potential intervention for sleep problems.
Trial registration: ClinicalTrials.gov NCT04953559; https://clinicaltrials.gov/ct2/show/NCT04953559.
International registered report identifier (irrid): DERR1-10.2196/51212.
Keywords: cTBS; continuous theta burst stimulation; default mode network; insomnia; randomized controlled trial; sleep; transcranial magnetic stimulation.
©Lindsey Hildebrand, Alisa Huskey, Natalie Dailey, Samantha Jankowski, Kymberly Henderson-Arredondo, Christopher Trapani, Salma Imran Patel, Allison Yu-Chin Chen, Ying-Hui Chou, William D S Killgore. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 26.01.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Morin CM, Vézina-Im LA, Ivers H, Micoulaud-Franchi JA, Philip P, Lamy M, Savard J. Prevalent, incident, and persistent insomnia in a population-based cohort tested before (2018) and during the first-wave of COVID-19 pandemic (2020) Sleep. 2022;45(1):zsab258. doi: 10.1093/sleep/zsab258. https://europepmc.org/abstract/MED/34698868 6410639 - DOI - PMC - PubMed
-
- AlRasheed MM, Fekih-Romdhane F, Jahrami H, Pires GN, Saif Z, Alenezi AF, Humood A, Chen W, Dai H, Bragazzi N, Pandi-Perumal SR, BaHammam AS, Vitiello MV. The prevalence and severity of insomnia symptoms during COVID-19: a global systematic review and individual participant data meta-analysis. Sleep Med. 2022;100:7–23. doi: 10.1016/j.sleep.2022.06.020. https://europepmc.org/abstract/MED/36030616 S1389-9457(22)01066-8 - DOI - PMC - PubMed
-
- Clancy F, Prestwich A, Caperon L, Tsipa A, O'Connor DB. The association between worry and rumination with sleep in non-clinical populations: a systematic review and meta-analysis. Health Psychol Rev. 2020;14(4):427–448. doi: 10.1080/17437199.2019.1700819. https://eprints.whiterose.ac.uk/154699/ - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

