Association of genetic variation in COL11A1 with adolescent idiopathic scoliosis
- PMID: 38277211
- PMCID: PMC10945706
- DOI: 10.7554/eLife.89762
Association of genetic variation in COL11A1 with adolescent idiopathic scoliosis
Abstract
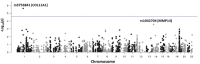
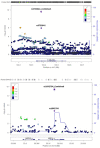
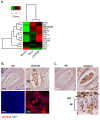
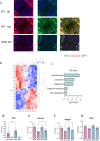
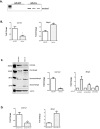
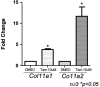
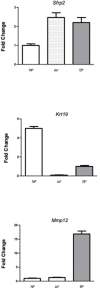
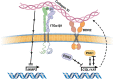
Adolescent idiopathic scoliosis (AIS) is a common and progressive spinal deformity in children that exhibits striking sexual dimorphism, with girls at more than fivefold greater risk of severe disease compared to boys. Despite its medical impact, the molecular mechanisms that drive AIS are largely unknown. We previously defined a female-specific AIS genetic risk locus in an enhancer near the PAX1 gene. Here, we sought to define the roles of PAX1 and newly identified AIS-associated genes in the developmental mechanism of AIS. In a genetic study of 10,519 individuals with AIS and 93,238 unaffected controls, significant association was identified with a variant in COL11A1 encoding collagen (α1) XI (rs3753841; NM_080629.2_c.4004C>T; p.(Pro1335Leu); p=7.07E-11, OR = 1.118). Using CRISPR mutagenesis we generated Pax1 knockout mice (Pax1-/-). In postnatal spines we found that PAX1 and collagen (α1) XI protein both localize within the intervertebral disc-vertebral junction region encompassing the growth plate, with less collagen (α1) XI detected in Pax1-/- spines compared to wild-type. By genetic targeting we found that wild-type Col11a1 expression in costal chondrocytes suppresses expression of Pax1 and of Mmp3, encoding the matrix metalloproteinase 3 enzyme implicated in matrix remodeling. However, the latter suppression was abrogated in the presence of the AIS-associated COL11A1P1335L mutant. Further, we found that either knockdown of the estrogen receptor gene Esr2 or tamoxifen treatment significantly altered Col11a1 and Mmp3 expression in chondrocytes. We propose a new molecular model of AIS pathogenesis wherein genetic variation and estrogen signaling increase disease susceptibility by altering a PAX1-COL11a1-MMP3 signaling axis in spinal chondrocytes.
Keywords: collagen XI; estrogen signaling; extracellular matrix; genetics; genomics; human; mouse; scoliosis.
Plain language summary
Adolescent idiopathic scoliosis (AIS) is a twisting deformity of the spine that occurs during periods of rapid growth in children worldwide. Children with severe cases of AIS require surgery to stop it from getting worse, presenting a significant financial burden to health systems and families. Although AIS is known to cluster in families, its genetic causes and its inheritance pattern have remained elusive. Additionally, AIS is known to be more prevalent in females, a bias that has not been explained. Advances in techniques to study the genetics underlying diseases have revealed that certain variations that increase the risk of AIS affect cartilage and connective tissue. In humans, one such variation is near a gene called Pax1, and it is female-specific. The extracellular matrix is a network of proteins and other molecules in the space between cells that help connect tissues together, and it is particularly important in cartilage and other connective tissues. One of the main components of the extracellular matrix is collagen. Yu, Kanshour, Ushiki et al. hypothesized that changes in the extracellular matrix could affect the cartilage and connective tissues of the spine, leading to AIS. To show this, the scientists screened over 100,000 individuals and found that AIS is associated with variants in two genes coding for extracellular matrix proteins. One of these variants was found in a gene called Col11a1, which codes for one of the proteins that makes up collagen. To understand the relationship between Pax1 and Col11a1, Yu, Kanshour, Ushiki et al. genetically modified mice so that they would lack the Pax1 gene. In these mice, the activation of Col11a1 was reduced in the mouse spine. They also found that the form of Col11a1 associated with AIS could not suppress the activation of a gene called Mmp3 in mouse cartilage cells as effectively as unmutated Col11a1. Going one step further, the researchers found that lowering the levels of an estrogen receptor altered the activation patterns of Pax1, Col11a1, and Mmp3 in mouse cartilage cells. These findings suggest a possible mechanism for AIS, particularly in females. The findings of Yu, Kanshour, Ushiki et al. highlight that cartilage cells in the spine are particularly relevant in AIS. The results also point to specific molecules within the extracellular matrix as important for maintaining proper alignment in the spine when children are growing rapidly. This information may guide future therapies aimed at maintaining healthy spinal cells in adolescent children, particularly girls.
© 2023, Yu, Khanshour, Ushiki et al.
Conflict of interest statement
HY, AK, AU, NO, YK, EE, YF, LA, YK, RC, RS, YZ, JP, NG, BE, JC, JH, CT, YS, CG, PG, SI, JR, NA, CW No competing interests declared
Figures





Update of
-
Association of genetic variation in COL11A1 with adolescent idiopathic scoliosis.bioRxiv [Preprint]. 2023 Nov 22:2023.05.26.542293. doi: 10.1101/2023.05.26.542293. bioRxiv. 2023. Update in: Elife. 2024 Jan 26;12:RP89762. doi: 10.7554/eLife.89762. PMID: 37292598 Free PMC article. Updated. Preprint.
References
-
- Ameur A, Dahlberg J, Olason P, Vezzi F, Karlsson R, Martin M, Viklund J, Kähäri AK, Lundin P, Che H, Thutkawkorapin J, Eisfeldt J, Lampa S, Dahlberg M, Hagberg J, Jareborg N, Liljedahl U, Jonasson I, Johansson Å, Feuk L, Lundeberg J, Syvänen A-C, Lundin S, Nilsson D, Nystedt B, Magnusson PK, Gyllensten U. SweGen: a whole-genome data resource of genetic variability in a cross-section of the Swedish population. European Journal of Human Genetics. 2017;25:1253–1260. doi: 10.1038/ejhg.2017.130. - DOI - PMC - PubMed
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. - DOI - PMC - PubMed
-
- Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Human Molecular Genetics. 2011;20:4041–4055. doi: 10.1093/hmg/ddr332. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

