Spatiotemporal control of pattern formation during somitogenesis
- PMID: 38277458
- PMCID: PMC10816718
- DOI: 10.1126/sciadv.adk8937
Spatiotemporal control of pattern formation during somitogenesis
Abstract
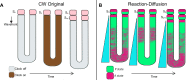
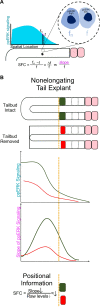
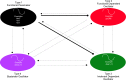
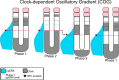
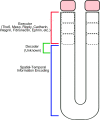
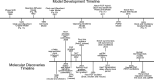
Spatiotemporal patterns widely occur in biological, chemical, and physical systems. Particularly, embryonic development displays a diverse gamut of repetitive patterns established in many tissues and organs. Branching treelike structures in lungs, kidneys, livers, pancreases, and mammary glands as well as digits and bones in appendages, teeth, and palates are just a few examples. A fascinating instance of repetitive patterning is the sequential segmentation of the primary body axis, which is conserved in all vertebrates and many arthropods and annelids. In these species, the body axis elongates at the posterior end of the embryo containing an unsegmented tissue. Meanwhile, segments sequentially bud off from the anterior end of the unsegmented tissue, laying down an exquisite repetitive pattern and creating a segmented body plan. In vertebrates, the paraxial mesoderm is sequentially divided into somites. In this review, we will discuss the most prominent models, the most puzzling experimental data, and outstanding questions in vertebrate somite segmentation.
Figures
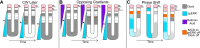
References
-
- Pourquie O., A brief history of the segmentation clock. Dev. Biol. 485, 24–36 (2022). - PubMed
-
- Piatkowska A. M., Evans S. E., Stern C. D., Cellular aspects of somite formation in vertebrates. Cells Dev. 168, 203732 (2021). - PubMed
-
- Gomez C., Ozbudak E. M., Wunderlich J., Baumann D., Lewis J., Pourquie O., Control of segment number in vertebrate embryos. Nature 454, 335–339 (2008). - PubMed
-
- Cooke J., Control of somite number during morphogenesis of a vertebrate, Xenopus laevis. Nature 254, 196–199 (1975). - PubMed
-
- Cooke J., Scale of body pattern adjusts to available cell number in amphibian embryos. Nature 290, 775–778 (1981). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

