Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML
- PMID: 38277619
- PMCID: PMC11095865
- DOI: 10.1200/JCO.23.01911
Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML
Abstract
Purpose: Azacitidine plus venetoclax is a standard of care for patients with newly diagnosed AML who are unfit for intensive chemotherapy. However, FLT3 mutations are a common mechanism of resistance to this regimen. The addition of gilteritinib, an oral FLT3 inhibitor, to azacitidine and venetoclax may improve outcomes in patients with FLT3-mutated AML.
Methods: This phase I/II study evaluated azacitidine, venetoclax, and gilteritinib in two cohorts: patients with (1) newly diagnosed FLT3-mutated AML who were unfit for intensive chemotherapy or (2) relapsed/refractory FLT3-mutated AML (ClinicalTrials.gov identifier: NCT04140487). The primary end points were the maximum tolerated dose of gilteritinib (phase I) and the combined complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate (phase II).
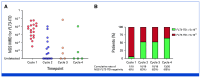
Results: Fifty-two patients were enrolled (frontline [n = 30]; relapsed/refractory [n = 22]). The recommended phase II dose was gilteritinib 80 mg once daily in combination with azacitidine and venetoclax. In the frontline cohort, the median age was 71 years and 73% of patients had an FLT3-internal tandem duplication (ITD) mutation. The CR/CRi rate was 96% (CR, 90%; CRi, 6%). Sixty-five percent of evaluable patients achieved FLT3-ITD measurable residual disease <5 × 10-5 within four cycles. With a median follow-up of 19.3 months, the median relapse-free survival (RFS) and overall survival (OS) have not been reached and the 18-month RFS and OS rates are 71% and 72%, respectively. In the relapsed/refractory cohort, the CR/CRi rate was 27%; nine additional patients (41%) achieved a morphologic leukemia-free state. The most common grade 3 or higher nonhematologic adverse events were infection (62%) and febrile neutropenia (38%), which were more frequent in the relapsed/refractory cohort.
Conclusion: The combination of azacitidine, venetoclax, and gilteritinib resulted in high rates of CR/CRi, deep FLT3 molecular responses, and encouraging survival in newly diagnosed FLT3-mutated AML. Myelosuppression was manageable with mitigative dosing strategies.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. - PubMed
-
- Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. - PubMed
-
- Janssen M, Schmidt C, Bruch PM, et al. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1. Blood. 2022;140:2594–2610. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

