Intracellular Synthesis of Indoles Enabled by Visible-Light Photocatalysis
- PMID: 38277674
- PMCID: PMC10859955
- DOI: 10.1021/jacs.3c13647
Intracellular Synthesis of Indoles Enabled by Visible-Light Photocatalysis
Abstract
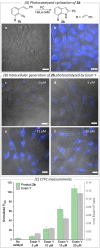
Performing abiotic synthetic transformations in live cell environments represents a new, promising approach to interrogate and manipulate biology and to uncover new types of biomedical tools. We now found that photocatalytic bond-forming reactions can be added to the toolbox of bioorthogonal synthetic chemistry. Specifically, we demonstrate that exogenous styryl aryl azides can be converted into indoles inside living mammalian cells under photocatalytic conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Johnston M. P.; Smith R. T.; MacMillan D. W. C. Visible Light Photocatalysis in Organic Synthesis. Angew. Chem., Int. Ed. 2016, 55, 15060–15078.
- Ravelli M.; Monti D.; Albini A. Photocatalysis in Organic Chemistry. Angew. Chem., Int. Ed. 2016, 55, 11902–11921.
- Marzo L.; Pagire S. K.; Reiser O.; König B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis?. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. 10.1002/anie.201709766. - DOI - PubMed
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. - DOI - PubMed
-
- Stephenson C. R. J.; Yoon T. P.; MacMillan D. W. C.. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Germany, 2018.
- Bonfield H. E.; Knauber T.; Lévesque F.; Moschetta E. G.; Susanne F.; Edwards L. J. Photons as a 21st century reagent. Nat. Commun. 2020, 11, 804.10.1038/s41467-019-13988-4. - DOI - PMC - PubMed
- Buzzetti L.; Crisenza G. E. M.; Melchiorre P. Mechanistic Studies in Photocatalysis. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. 10.1002/anie.201809984. - DOI - PubMed
- Wang H.; Tian Y. M.; König B. Energy- and atom-efficient chemical synthesis with endergonic photocatalysis. Nat. Rev. Chem. 2022, 6, 745–755. 10.1038/s41570-022-00421-6. - DOI - PubMed
- Strieth-Kalthoff F.; Glorius F. Triplet Energy Transfer Photocatalysis: Unlocking the Next Level. Chem. 2020, 6, 1888.10.1016/j.chempr.2020.07.010. - DOI
-
- Wang H.; Li W.-G.; Zeng K.; Wu Y.-J.; Zhang Y.; Xu T.-L.; Chen Y. Photocatalysis Enables Visible-Light Uncaging of Bioactive Molecules in Live Cells. Angew. Chem., Int. Ed. 2019, 58, 561–656. 10.1002/anie.201811261. - DOI - PubMed
- Li M.; Gebremedhin K. H.; Ma D.; Pu Z.; Xiong T.; Xu Y.; Kim J. S.; Peng X. Conditionally Activatable Photoredox catalysis in Living Systems. J. Am. Chem. Soc. 2022, 144, 163–173. 10.1021/jacs.1c07372. - DOI - PubMed
- Zeng K.; Han L.; Chen Y. Endogenous Proteins Modulation in Live Cells with Small Molecules and Light. ChemBioChem. 2022, 23, e20220024410.1002/cbic.202200244. - DOI - PubMed
- Alonso-de Castro S.; Ruggiero E.; Ruiz-de-Angulo A.; Rezabal E.; Mareque-Rivas J. C.; Lopez X.; Lopez-Gallego F.; Salassa L. Riboflavin as bioorthogonal photocatalyst for the activation of a PtIV prodrug. Chem. Sci. 2017, 8, 4619–4625. 10.1039/C7SC01109A. - DOI - PMC - PubMed
-
- Liu W.; Watson E. E.; Winssinger N. Photocatalysis in Chemical Biology: Extending the Scope of Optochemical Control and Towards New Frontiers in Semisynthetic Bioconjugates and Biocatalysis. Helv. Chim. Acta 2021, 104, e20210017910.1002/hlca.202100179. - DOI
- Angerani S.; Winssinger N. Visible Light Photoredox Catalysis Using Ruthenium Complexes in Chemical Biology. Chem.—Eur. J. 2019, 25, 6661–6672. 10.1002/chem.201806024. - DOI - PubMed
- Meggers E. Photocatalysis in organic and medicinal chemistry. Topics in Current Chemistry 2018, 376, 3. - PubMed
- Madec H.; Figueiredo F.; Cariou K.; Roland S.; Sollogoub M.; Gasser G. Metal complexes for catalytic and photocatalytic reactions in living cells and organisms. Chem. Sci. 2023, 14, 409–442. 10.1039/D2SC05672K. - DOI - PMC - PubMed
- Interrogating Biological Systems Using Visible-Light-Powered Catalysis. Nat. Rev. Chem. 2021, 5, 322–337.10.1038/s41570-021-00265-6 - DOI - PubMed
- Li P.; Terrett J. A.; Zbieg J. R. Visible-Light Photocatalysis as an Enabling Technology for Drug Discovery: A Paradigm Shift for Chemical Reactivity. ACS Med. Chem. Lett. 2020, 11, 2120–2130. 10.1021/acsmedchemlett.0c00436. - DOI - PMC - PubMed
- Fang Y.; Zou P. Photocatalytic Proximity Labeling for Profiling the Subcellular Organization of Biomolecules. ChemBioChem. 2023, 24, e20220074510.1002/cbic.202200745. - DOI - PubMed
- Seath C. P.; Trowbridge A. D.; Muir T. W.; MacMillan D. W. Reactive intermediates for interactome mapping. Chem. Soc. Rev. 2021, 50, 2911–2926. 10.1039/D0CS01366H. - DOI - PubMed
LinkOut - more resources
Full Text Sources

