Proinflammatory phenotype of iPS cell-derived JAK2 V617F megakaryocytes induces fibrosis in 3D in vitro bone marrow niche
- PMID: 38278152
- PMCID: PMC10874863
- DOI: 10.1016/j.stemcr.2023.12.011
Proinflammatory phenotype of iPS cell-derived JAK2 V617F megakaryocytes induces fibrosis in 3D in vitro bone marrow niche
Abstract
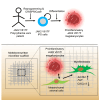
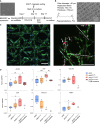
The myeloproliferative disease polycythemia vera (PV) driven by the JAK2 V617F mutation can transform into myelofibrosis (post-PV-MF). It remains an open question how JAK2 V617F in hematopoietic stem cells induces MF. Megakaryocytes are major players in murine PV models but are difficult to study in the human setting. We generated induced pluripotent stem cells (iPSCs) from JAK2 V617F PV patients and differentiated them into megakaryocytes. In differentiation assays, JAK2 V617F iPSCs recapitulated the pathognomonic skewed megakaryocytic and erythroid differentiation. JAK2 V617F iPSCs had a TPO-independent and increased propensity to differentiate into megakaryocytes. RNA sequencing of JAK2 V617F iPSC-derived megakaryocytes reflected a proinflammatory, profibrotic phenotype and decreased ribosome biogenesis. In three-dimensional (3D) coculture, JAK2 V617F megakaryocytes induced a profibrotic phenotype through direct cell contact, which was reversed by the JAK2 inhibitor ruxolitinib. The 3D coculture system opens the perspective for further disease modeling and drug discovery.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests W.W. and V.T. are involved in Cygenia GmbH (www.cygenia.com), which provides services for DNA methylation analysis to other scientists. S.K. reports funding from Novartis, Bristol-Myers Squibb, and Janssen/Geron; advisory board honoraria from Pfizer, Incyte, Ariad, Novartis, AOP Pharma, BMS, Celgene, Geron, Janssen, CTI, Roche, Baxalta, GSK, Sierra Oncology, and Sanofi; a patent for Bromodomain and Extra-Terminal (BET) inhibitor at RWTH Aachen University; honoraria from Novartis, Bristol Myers Squibb, Celgene, Geron, Janssen, Pfizer, Incyte, Ariad, Shire, Roche, and AOP Pharma; and other financial support (e.g., travel support) from Alexion, Novartis, Bristol Myers Squibb, Incyte, Ariad, AOP Pharma, Baxalta, CTI, Pfizer, Sanofi, Celgene, Shire, Janssen, Geron, Abbvie, Imago Biosciences, Sierra Oncology, GSK, and Karthos. T.H.B. served as a consultant for Janssen, Merck, Novartis, and Pfizer; received research funding form Novartis and Pfizer; and received honoraria from Janssen, Merck, Novartis, and Pfizer.
Figures




References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. - PubMed
-
- Balduini A., Badalucco S., Pugliano M.T., Baev D., De Silvestri A., Cattaneo M., Rosti V., Barosi G. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS One. 2011;6:e21015. - PMC - PubMed
-
- Baumeister J., Chatain N., Hubrich A., Maié T., Costa I.G., Denecke B., Han L., Küstermann C., Sontag S., Seré K., et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34:1062–1074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

