The transcription factor AP2XI-2 is a key negative regulator of Toxoplasma gondii merogony
- PMID: 38278808
- PMCID: PMC10817966
- DOI: 10.1038/s41467-024-44967-z
The transcription factor AP2XI-2 is a key negative regulator of Toxoplasma gondii merogony
Abstract
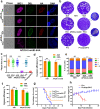
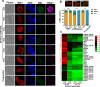
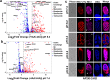
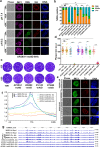
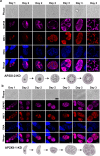
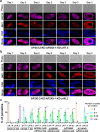
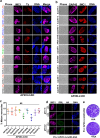
Sexual development in Toxoplasma gondii is a multistep process that culminates in the production of oocysts, constituting approximately 50% of human infections. However, the molecular mechanisms governing sexual commitment in this parasite remain poorly understood. Here, we demonstrate that the transcription factors AP2XI-2 and AP2XII-1 act as negative regulators, suppressing merozoite-primed pre-sexual commitment during asexual development. Depletion of AP2XI-2 in type II Pru strain induces merogony and production of mature merozoites in an alkaline medium but not in a neutral medium. In contrast, AP2XII-1-depleted Pru strain undergoes several rounds of merogony and produces merozoites in a neutral medium, with more pronounced effects observed under alkaline conditions. Additionally, we identified two additional AP2XI-2-interacting proteins involved in repressing merozoite programming. These findings underscore the intricate regulation of pre-sexual commitment by a network of factors and suggest that AP2XI-2 or AP2XII-1-depleted Pru parasites can serve as a model for studying merogony in vitro.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

