Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib - interim results from the DAstop2 trial
- PMID: 38278960
- PMCID: PMC10997502
- DOI: 10.1038/s41375-024-02145-6
Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib - interim results from the DAstop2 trial
Erratum in
-
Correction: Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib - interim results from the DAstop2 trial.Leukemia. 2024 Apr;38(4):925. doi: 10.1038/s41375-024-02184-z. Leukemia. 2024. PMID: 38418611 Free PMC article. No abstract available.
Abstract
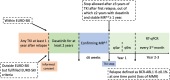
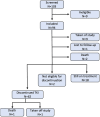
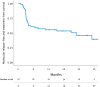
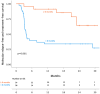
Tyrosine kinase inhibitor (TKI) discontinuation in chronic myeloid leukemia (CML) has become part of routine care for patients with a sustained deep molecular response (DMR). Approximately 50% experience a molecular relapse upon TKI cessation. Most of them quickly regain DMR upon TKI resumption. Whether these patients can achieve a second treatment-free remission (TFR) remains unclear. DAstop2 (ClinicalTrials.gov ID: NCT03573596) is a prospective study including patients with a failed first TFR attempt re-treated with any TKI for ≥ one year. Upon entering the study, patients received the TKI dasatinib for additional two years. Patients with sustained DMR for ≥1 year qualified for a second TKI stop. Ninety-four patients were included between Oct 2017-Dec 2021. At the time of data analysis, 62 patients had attempted a 2nd stop. After a median follow-up of 27 months from 2nd stop, TFR rates were 61, 56 and 46% at 6, 12 and 24 months respectively. No progression to advanced stage disease was seen and 87% had re-achieved MR4 within a median of 3 months from TKI re-initiation. In summary, we show that a 2nd TFR attempt after dasatinib treatment is safe, feasible and TFR rates seem in the range of those reported in trials of a first TKI stop.
© 2024. The Author(s).
Conflict of interest statement
Study drug was provided by BMS. DW have received study support and honoraria from BMS, Novartis, Pfizer, Austrian Orphan Pharma and Incyte. SS have received honoraria from BMS, Novartis, Incyte, Roche and research support from Incyte and Novartis. In addition, the Nordic CML Study group have other projects supported by Incyte, Pfizer and Austrian Orphan Pharma.
Figures
References
-
- Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. The Lancet Oncology. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

